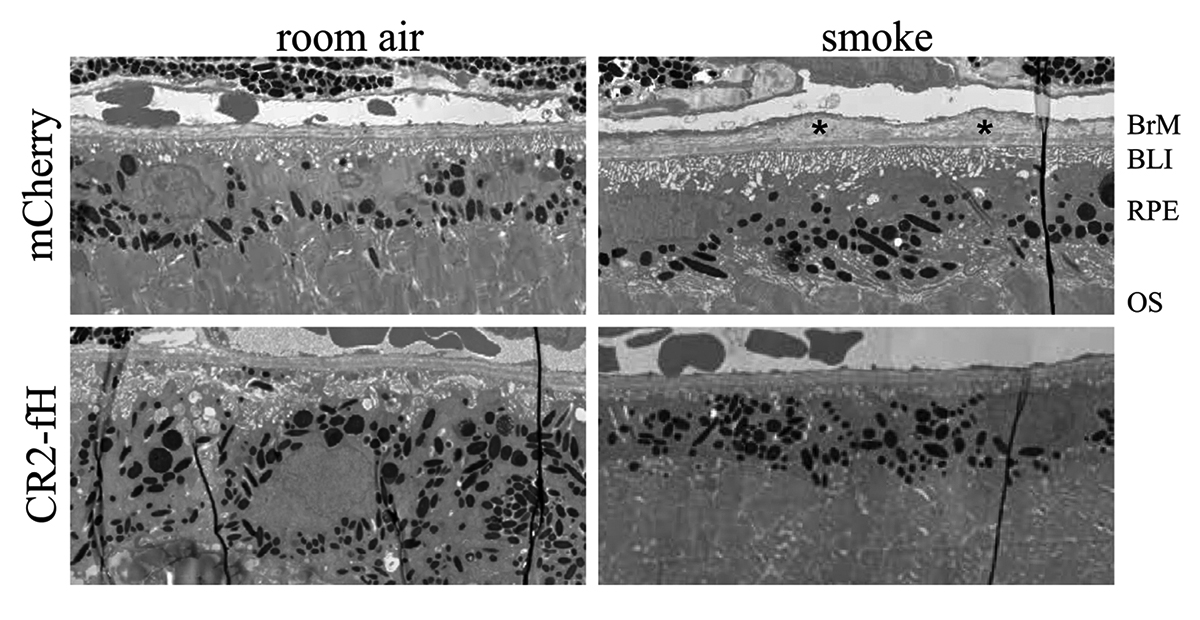
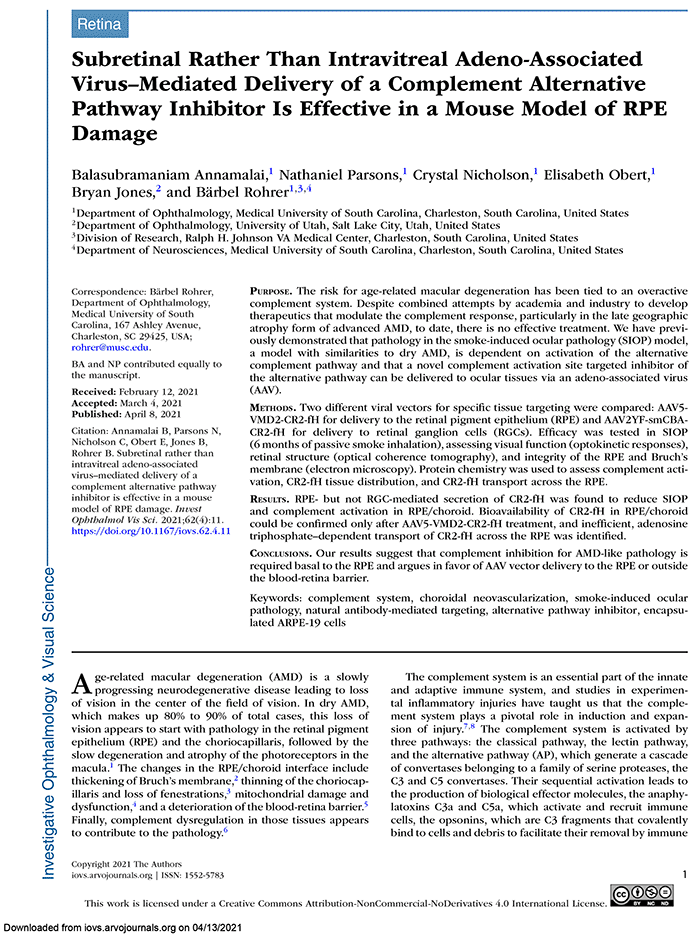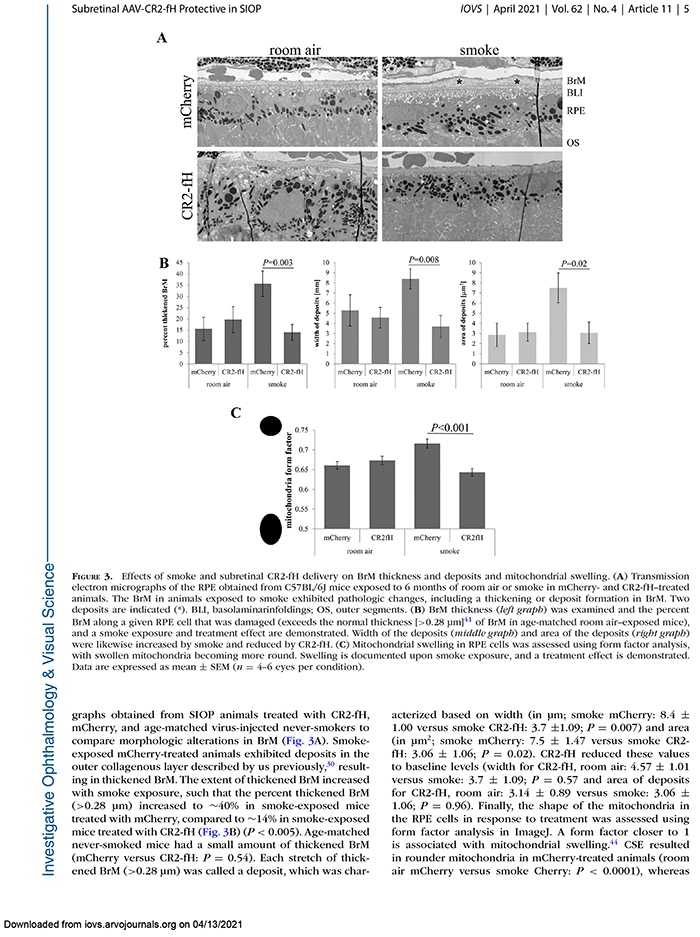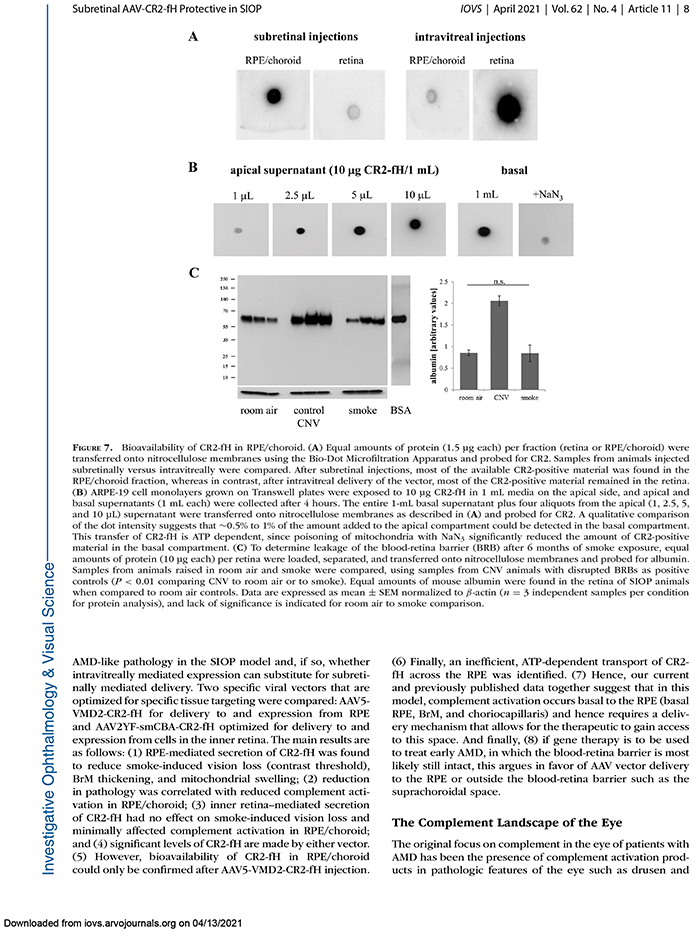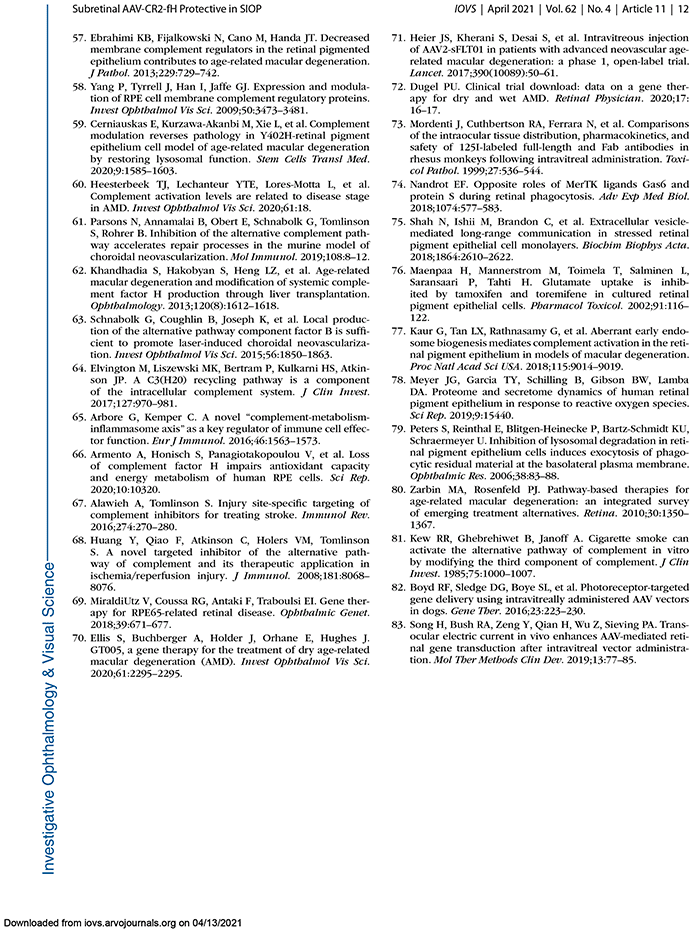
We have a new manuscript out in iOVS, Subretinal Rather Than Intravitreal Adeno-Associated Virus–Mediated Delivery of a Complement Alternative Pathway Inhibitor Is Effective in a Mouse Model of RPE Damage. (pdf here)
Authors: Balasubramaniam Annamalai; Nathaniel Parsons; Crystal Nicholson; Elisabeth Obert; Bryan W. Jones @BWJones; and Bärbel Rohrer.
Abstract:
Purpose: The risk for age-related macular degeneration has been tied to an overactive complement system. Despite combined attempts by academia and industry to develop therapeutics that modulate the complement response, particularly in the late geographic atrophy form of advanced AMD, to date, there is no effective treatment. We have previously demonstrated that pathology in the smoke-induced ocular pathology (SIOP) model, a model with similarities to dry AMD, is dependent on activation of the alternative complement pathway and that a novel complement activation site targeted inhibitor of the alternative pathway can be delivered to ocular tissues via an adeno-associated virus (AAV).
Methods: Two different viral vectors for specific tissue targeting were compared: AAV5-VMD2-CR2-fH for delivery to the retinal pigment epithelium (RPE) and AAV2YF-smCBA-CR2-fH for delivery to retinal ganglion cells (RGCs). Efficacy was tested in SIOP (6 months of passive smoke inhalation), assessing visual function (optokinetic responses), retinal structure (optical coherence tomography), and integrity of the RPE and Bruch’s membrane (electron microscopy). Protein chemistry was used to assess complement activation, CR2-fH tissue distribution, and CR2-fH transport across the RPE.
Results: RPE- but not RGC-mediated secretion of CR2-fH was found to reduce SIOP and complement activation in RPE/choroid. Bioavailability of CR2-fH in RPE/choroid could be confirmed only after AAV5-VMD2-CR2-fH treatment, and inefficient, adenosine triphosphate–dependent transport of CR2-fH across the RPE was identified.
Conclusions: Our results suggest that complement inhibition for AMD-like pathology is required basal to the RPE and argues in favor of AAV vector delivery to the RPE or outside the blood-retina barrier.