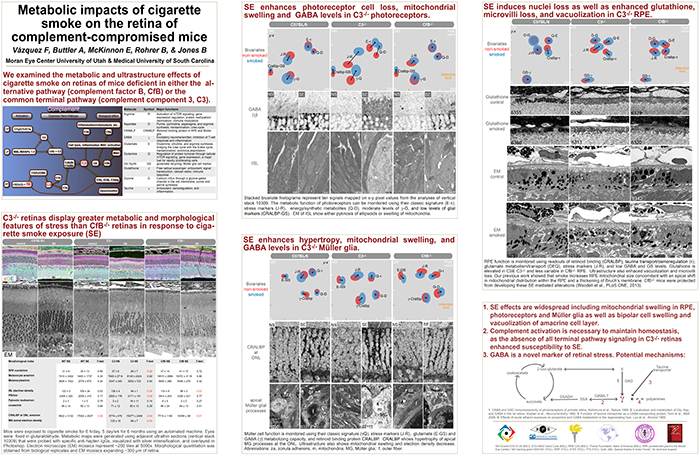
This abstract was presented today, May 8th at the 2017 Association for Research in Vision and Opthalmology (ARVO) meetings in Baltimore, Maryland by Felix Vazquez-Chona, Alex Butler, Emile McKinnon, Baerbel Rohrer, and Bryan W. Jones. Full resolution version here.
Purpose: The interaction between metabolism and the immune system is hypothesized as playing a central role in the pathology of neural diseases including Age-Related Macular Degeneration (AMD). We investigated the effects of cigarette-smoke exposure (CSE) on metabolism of retinal cells in wild-type (wt) mice, and mice deficient for the alternative pathway (complement factor B, CfB) or common terminal pathway (complement component 3, C3).
Methods: Mice were exposed to CSE or room filtered-air (controls) for 6 h/d, 5 d/wk for 6 months. We visualized the metabolism of retinal cells using Computational Molecular Phenotyping (CMP). Retinal cell classification and metabolic adaptation were interrogated using arginine (R), aspartate (D), GABA (γ), glutamate (E), glycine (G), glutathione (J), glutamine (Q), taurine (τ), glutamine synthetase (GS), and cellular retinaldehyde binding protein (CRALBP). Electron microscope mosaics were instrumental in phenotyping metabolic profiles.
Results: CSE C3-/- animals show more severe degenerative indices than CSE WT: retinal pigment epithelium (RPE) exhibited a decreased basalateral infolding area and increased vacuolization; photoreceptors show increased mitochondrial swelling and pyknosis; Müller glia displayed hypertrophy; and the amacrine layer was affected by increased vacuolization. The CfB-/- retina was more resilient to the negative effects of CSE when compared to the WT retina. At the metabolic level, RPE and inner segments of CSE CfB-/- mice displayed modest changes. In contrast, changes in CSE C3-/- and WT retina were dramatic: RPE exhibited decreased CRALBP and elevated R-E-J-τ-γ levels; inner segments showed increased R-D-E-G-J-Q-τ-γ-CRALBP; and Müller glia were found to have decreased levels along the R-D-E-G-J-Q-τ-γ-GS-CRALBP axis.
Conclusions: Increased GABA levels in RPE and photoreceptors are consistent with Müller glia dysfunction. Our metabolic profiling suggests that RPE and Müller glia are vulnerable to CSE-induced oxidative stress. We also find that the potential complement activation status of the retina-RPE-choroid unit highly influences the metabolic response of retinal cells to CSE. As complete blockade of the complement system in the C3-/- model has a more dramatic impact on metabolism of RPE, Müller glia, and photoreceptors than observed in the CfB-/- model, it can be proposed that downstream signaling of the complement system is required for retina health.
Layman Abstract: Metabolism involves a complex circuitry of metabolic pathways, intermediates, and cell-cell interactions. Thus, mapping metabolism with cellular resolution and quantitative power is key to identifying robust biomarkers of disease progression.