We have a new publication out as collaborators with colleages, Store-Operated Calcium Entry In Müller Glia Is Controlled By Synergistic Activation Of TRPC And Orai Channels authored by Tünde Molnár, Oleg Yarishkin, Peter Barabas, Anthony Iuso, Bryan W. Jones, Robert Marc, Tam Phuong, and David Krizaj.
Bonus, we got the cover! The image was created by Tam Phuong.
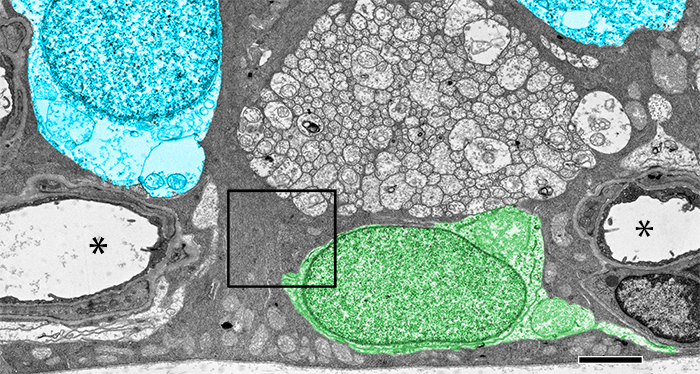
Significance: Store-operated Ca2+ signaling represents a major signaling pathway and source of cytosolic Ca2+ in astrocytes. Here, we show that the store-operated response in Müller cells, radial glia that perform key structural, signaling, osmoregulatory and mechanosensory functions within the retina, is mediated through synergistic activation of TRPC and Orai channels. The endfoot disproportionately expresses the depletion sensor STIM1, contains an extraordinarily high density of ER cisternae that shadow neuronal, astrocyte, vascular and axonal structures, interface with mitochondria but also originates SOCE-induced transcellular Ca2+ waves that propagate glial excitation into the proximal retina. These results identify a molecular mechanism that underlies complex interactions between the plasma membrane and calcium stores and contributes to radial glial function, regulation and response to mechanical stress.
Abstract: The endoplasmic reticulum (ER) is at the epicenter of astrocyte Ca2+ signaling. We sought to identify the molecular mechanism underlying store-operated calcium entry (SOCE) that repletes ER Ca2+ stores in mouse Müller cells. Store depletion, induced through blockade of sequestration transporters in Ca2+-free saline, induced synergistic activation of canonical transient receptor potential (TRPC1) and Orai channels. Store-operated TRPC1 channels were identified by their electrophysiological properties, pharmacological blockers and ablation of the Trpc1 gene. ICRAC (Ca2+ release-activated) currents were identified by ion permeability, voltage-dependence and sensitivity to selective Orai antagonists Synta66 and GSK7975A. Depletion-evoked calcium influx was initiated at the Müller endfoot and apical process, triggering centrifugal propagation of Ca2+ waves into the cell body. EM analysis of the endfoot compartment showed high-density ER cisternae that shadow retinal ganglion cell (RGC) somata and axons, protoplasmic astrocytes, vascular endothelial cells and ER-mitochondrial contacts at the vitreal surface of the endfoot. The mouse retina expresses transcripts encoding both Stim and all known Orai genes; Müller glia predominantly express STIM1 whereas STIM2 is mainly confined to the outer plexiform and retinal ganglion cell layers. Elimination of TRPC1 facilitated Müller gliosis induced by the elevation of intraocular pressure (IOP), suggesting that TRPC channels might play a neuroprotective role during mechanical stress. These findings expand the current knowledge about store-operated signaling in astroglia, as well as calcium signaling pathways in Müller astroglia and functional roles these cells play in retinal physiology and pathology.