We are pleased to reveal that Dr. Bryan William Jones has been selected for an RPB Stein Innovation Award from Research to Prevent Blindness. This particular project is something that we’ve been scheming for a while and leverages an approach to comparative anatomy to study the ground squirrel retina. The unique thing about the 12-lined ground squirrel retina is that the photoreceptors of this organism degenerate when it hibernates. The outer segments of the photoreceptors degenerate and the synapses that connect them to the first synapse of the visual system dissolves in much the same way as when the retina degenerates in human diseases like retinitis pigmentosa, and age-related macular degeneration. The trick is: When the 13-lined ground squirrel comes out of hibernation, their retinas regenerate and their synapses reconnect giving us an incredible opportunity to explore plasticity in their nervous systems.
Dr. Rebecca Pfeiffer Awarded RPB Career Development Award
Dr. Rebecca Pfeiffer from our team has just been awarded a Research to Prevent Blindness (RPB) Career Development Award to explore how Müller glia interact with synapses at the network level. We are so incredibly proud of her and are looking forward to seeing where her science develops as a result of this award. Our immense gratitude to RPB for all of their support over the years as without them, the science that comes out of this laboratory would not be possible.
Bryan William Jones TEDx Berlin Talk: How Your Vision Determines Your Reality
In February, lab PI Bryan William Jones traveled to Berlin to give a talk for TEDx Berlin. Now the full talk from his presentation at TEDx Berlin has been posted.
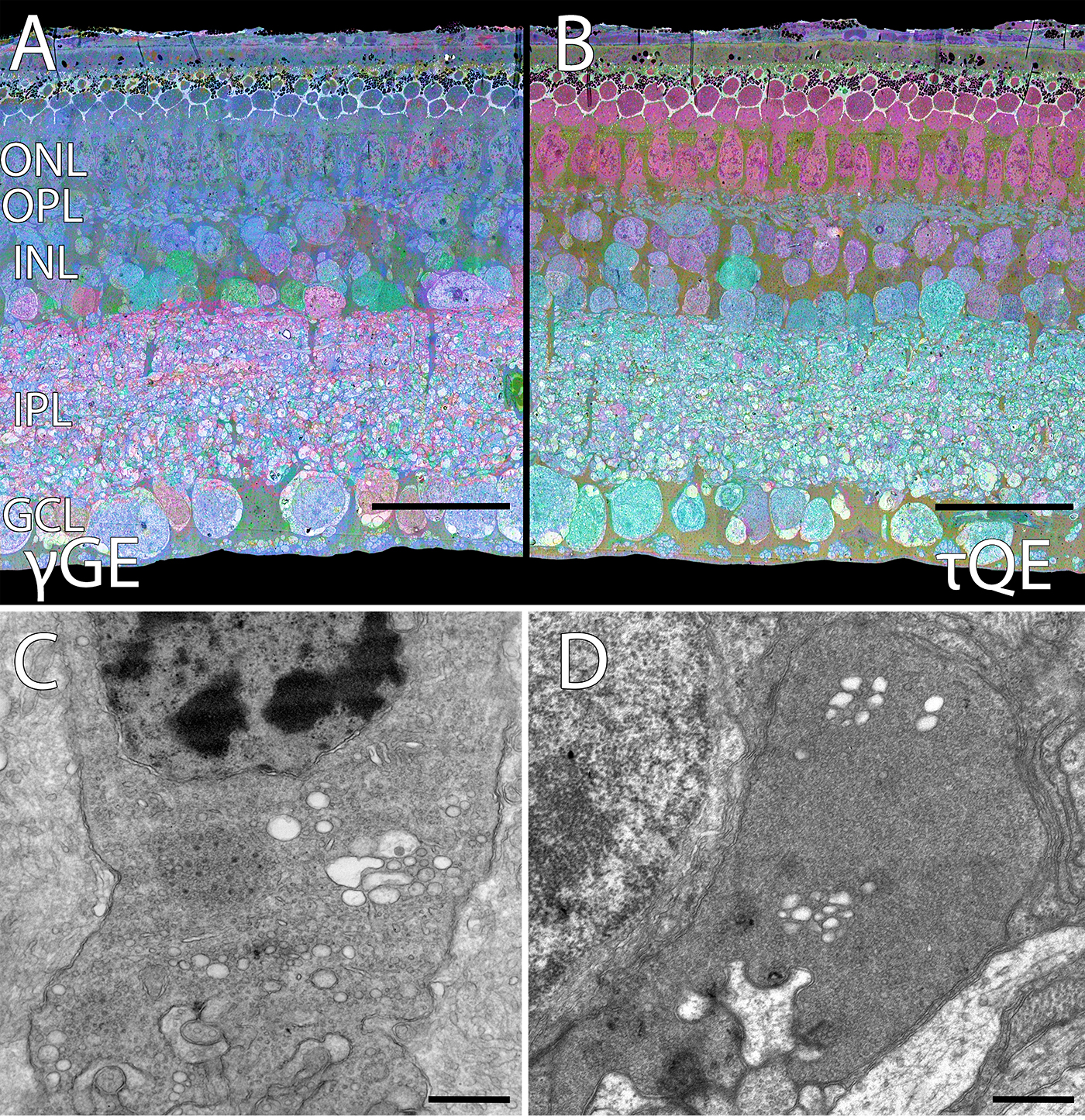
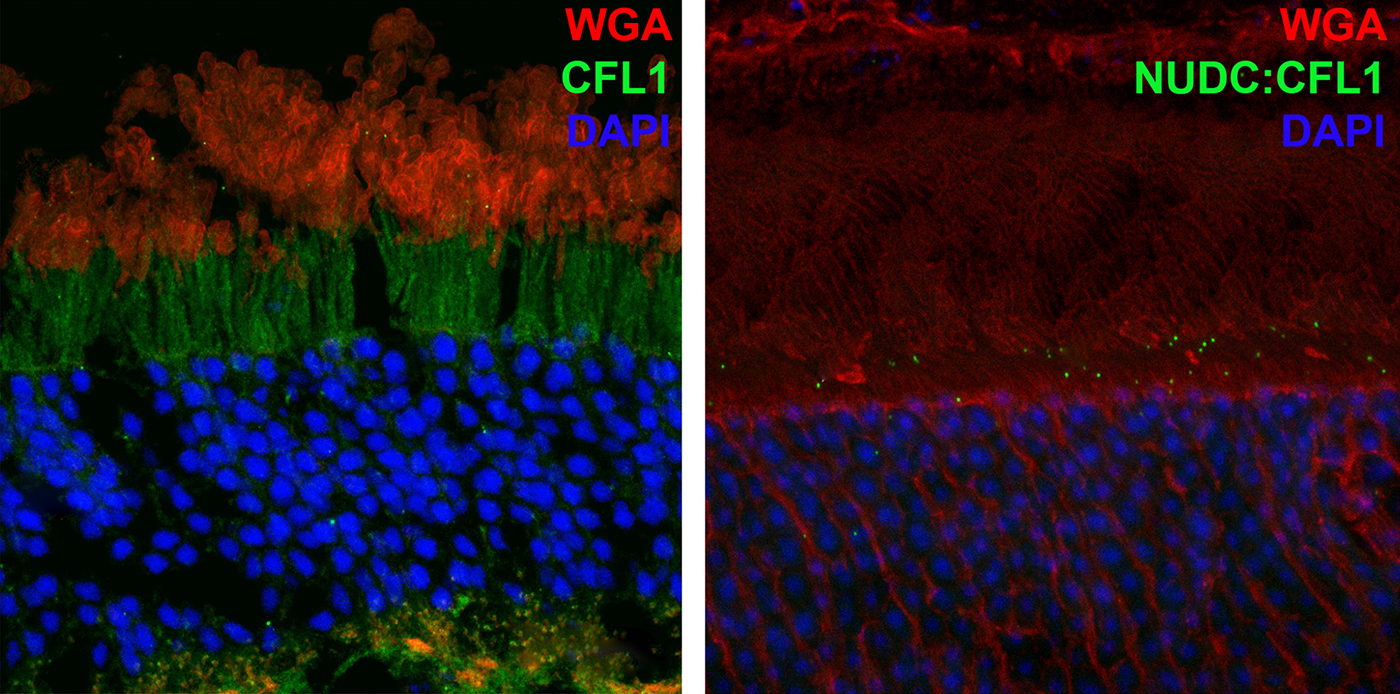
NUDC Is Critical For Rod Photoreceptor Function, Maintenance, And Survival
We have a new manuscript in collaboration with the Gross Lab out of UAB in The FASEB Journal, (PubMed link here): NUDC is critical for rod photoreceptor function, maintenance, and survival. This manuscript is in collaboration with. Authors are: Mary Anne Garner, Meredith G. Hubbard, Evan R. Boitet, Seth T. Hubbard, Anushree Gade, Guoxin Ying, Bryan W. Jones, Wolfgang Baehr, Alecia K. Gross. The PDF is here.
Abstract: NUDC (nuclear distribution protein C) is a mitotic protein involved in nuclear migration and cytokinesis across species. Considered a cytoplasmic dynein (henceforth dynein) cofactor, NUDC was shown to associate with the dynein motor complex during neuronal migration. NUDC is also expressed in postmitotic vertebrate rod photoreceptors where its function is unknown. Here, we examined the role of NUDC in postmitotic rod photoreceptors by studying the consequences of a conditional NUDC knockout in mouse rods (rNudC−/−). Loss of NUDC in rods led to complete photoreceptor cell death at 6 weeks of age. By 3 weeks of age, rNudC−/− function was diminished, and rhodopsin and mitochondria were mislocalized, consistent with dynein inhibition. Levels of outer segment proteins were reduced, but LIS1 (lissencephaly protein 1), a well-characterized dynein cofactor, was unaffected. Transmission electron microscopy revealed ultrastructural defects within the rods of rNudC−/− by 3 weeks of age. We investigated whether NUDC interacts with the actin modulator cofilin 1 (CFL1) and found that in rods, CFL1 is localized in close proximity to NUDC. In addition to its potential role in dynein trafficking within rods, loss of NUDC also resulted in increased levels of phosphorylated CFL1 (pCFL1), which would purportedly prevent depolymerization of actin. The absence of NUDC also induced an inflammatory response in Müller glia and microglia across the neural retina by 3 weeks of age. Taken together, our data illustrate the critical role of NUDC in actin cytoskeletal maintenance and dynein-mediated protein trafficking in a postmitotic rod photoreceptor.
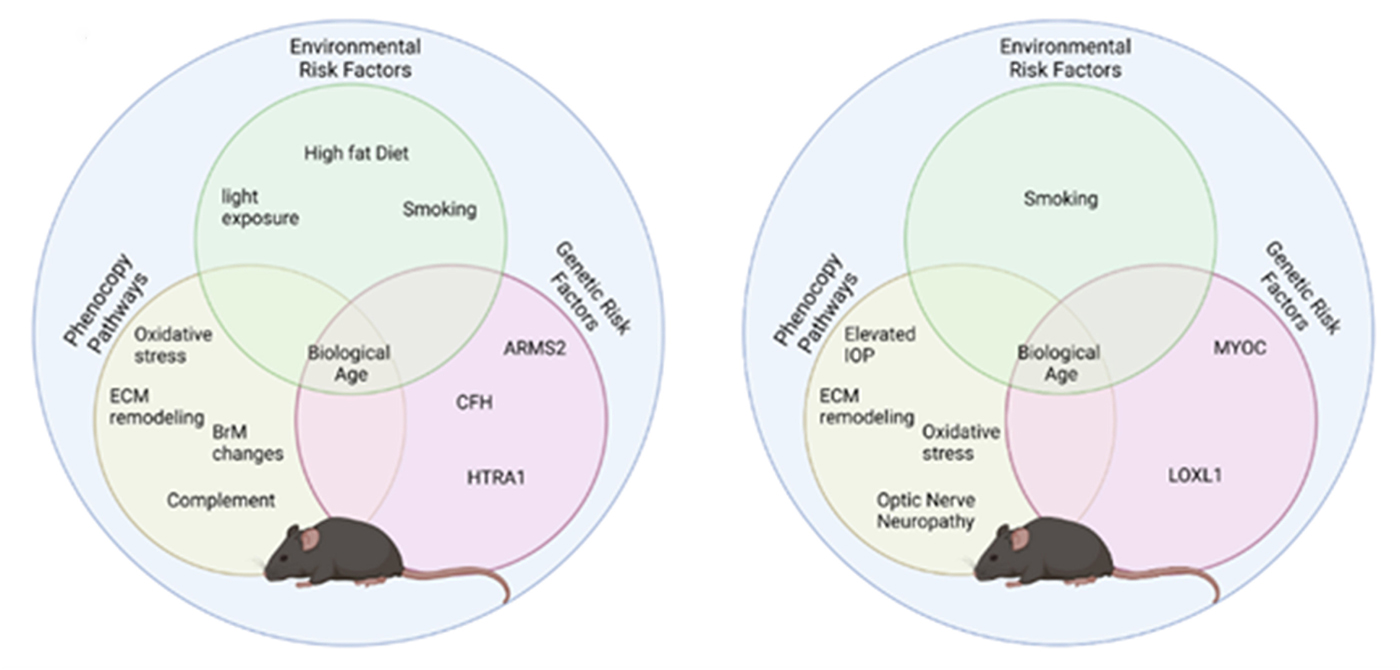
Modeling Complex Age-Related Eye Disease
We have a new Progress in Retinal and Eye Research manuscript out in collaboration with my colleagues here at the Moran Eye Center.
Authors: Silke Becker, Zia L’Ecuyer, Bryan W Jones, Moussa A Zouache, Fiona S McDonnell, Frans Vinberg.
Abstract: Modeling complex eye diseases like age-related macular degeneration (AMD) and glaucoma poses significant challenges, since these conditions depend highly on age-related changes that occur over several decades, with many contributing factors remaining unknown. Although both diseases exhibit a relatively high heritability of >50%, a large proportion of individuals carrying AMD- or glaucoma-associated genetic risk variants will never develop these diseases. Furthermore, several environmental and lifestyle factors contribute to and modulate the pathogenesis and progression of AMD and glaucoma.
Several strategies replicate the impact of genetic risk variants, pathobiological pathways and environmental and lifestyle factors in AMD and glaucoma in mice and other species. In this review we will mostly discuss the most commonly available mouse models, which have and will likely continue to improve our understanding of the pathobiology of age-related eye diseases. Uncertainties persist whether small animal models can truly recapitulate disease progression and vision loss in patients, raising doubts regarding their usefulness when testing novel gene or drug therapies. We will elaborate on concerns that relate to shorter lifespan, body size and allometries, lack of macula and a true lamina cribrosa, as well as absence and sequence disparities of certain genes and differences in their chromosomal location in mice.
Since biological, rather than chronological, age likely predisposes an organism for both glaucoma and AMD, more rapidly aging organisms like small rodents may open up possibilities that will make research of these diseases more timely and financially feasible. On the other hand, due to the above-mentioned anatomical and physiological features, as well as pharmacokinetic and -dynamic differences small animal models are not ideal to study the natural progression of vision loss or the efficacy and safety of novel therapies. In this context, we will also discuss the advantages and pitfalls of alternative models that include larger species, such as non-human primates and rabbits, patient-derived retinal organoids, and human organ donor eyes.
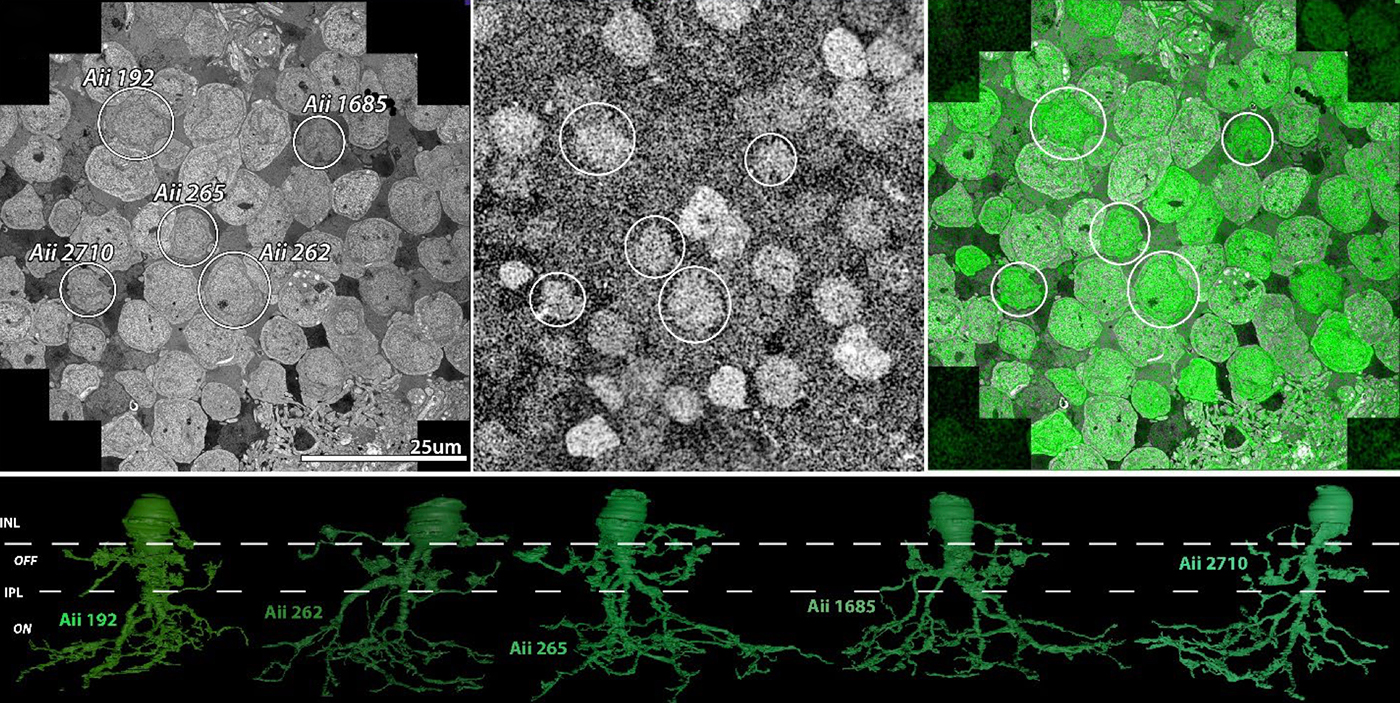
Preprint: Neural Circuit Revision in Retinal Remodeling, A Pathoconnectomics Approach
We have a new preprint out, Neural Circuit Revision in Retinal Remodeling, A Pathoconnectomics Approach.
Authors: Rebecca L Pfeiffer, Jeebika Dahal, Crystal L Sigulinsky, James R Anderson, Isabel A Barrera, Jia-Hui Yang, Olivia Haddadin, Alexis R Houser, Jessica C Garcia, Bryan William Jones
Abstract: The Aii glycinergic amacrine cell (Aii) plays a central role in bridging rod pathways with cone pathways, enabling an increased dynamic range of vision from scotopic to photopic ranges. The Aii integrates scotopic signals via chemical synapses from rod bipolar cells (RodBCs) onto the arboreal processes of Aii ACs, injecting signals into ON-cone bipolar cells (CBbs) via gap junctions with Aiis on the arboreal processes and the waist of the Aii ACs. The CBbs then carry this information to ON and OFF ganglion cell classes. In addition, the Aii is involved in the surround inhibition of OFF cone bipolar cells (CBas) through glycinergic chemical synapses from Aii ACs onto CBas. We have previously shown changes in RodBC connectivity as a consequence of rod photoreceptor degeneration in a pathoconnectome of early retinal degeneration: RPC1. Here, we evaluated the impact of rod photoreceptor degeneration on the connectivity of the Aii to determine the impacts of photoreceptor degeneration on the downstream network of the neural retina and its suitability for integrating therapeutic interventions as rod photoreceptors are lost. Previously, we reported that in early retinal degeneration, prior to photoreceptor cell loss, Rod BCs make pathological gap junctions with Aiis. Here, we further characterize this altered connectivity and additional shifts in both the excitatory drive and gap junctional coupling of Aiis in retinal degeneration, along with discussion of the broader impact of altered connectivity networks. New findings reported here demonstrate that Aiis make additional gap junctions with CBas increasing the number of BC classes that make pathological gap junctional connectivity with Aiis in degenerating retina. In this study, we also report that the Aii, a tertiary retinal neuron alters their synaptic contacts early in photoreceptor degeneration, indicating that rewiring occurs in more distant members of the retinal network earlier in degeneration than was previously predicted. This rewiring impacts retinal processing, presumably acuity, and ultimately its ability to support therapeutics designed to restore image-forming vision. Finally, these Aii alterations may be the cellular network level finding that explains one of the first clinical complaints from human patients with retinal degenerative disease, an inability to adapt back and forth from photopic to scotopic conditions.
UCI Center For Translational Vision Research Talk on Retinal Connectomics and Pathoconnectomics
Unbalanced Redox Status Network As An Early Pathological Event In Congenital Cataracts
We have a new manuscript from the lab in Experimental Eye Research, (PubMed link here). Metabolic changes and retinal remodeling in Heterozygous CRX mutant cats (CRXRDY/+). This manuscript is in collaboration with the Sheldon Rowan lab out of Tufts University. Authors are: Eloy Bejarano, Elizabeth A Whitcomb, Rebecca L Pfeiffer (@BeccaPfeiffer19), Kristie L Rose, Maria José Asensio, José Antonio Rodríguez-Navarro, Alejandro Ponce-Mora, Antolín Canto, Inma Almansa, Kevin L Schey, Bryan W Jones (@BWJones), Allen Taylor, and Sheldon Rowan (@SheldonRowan). The PDF is here.
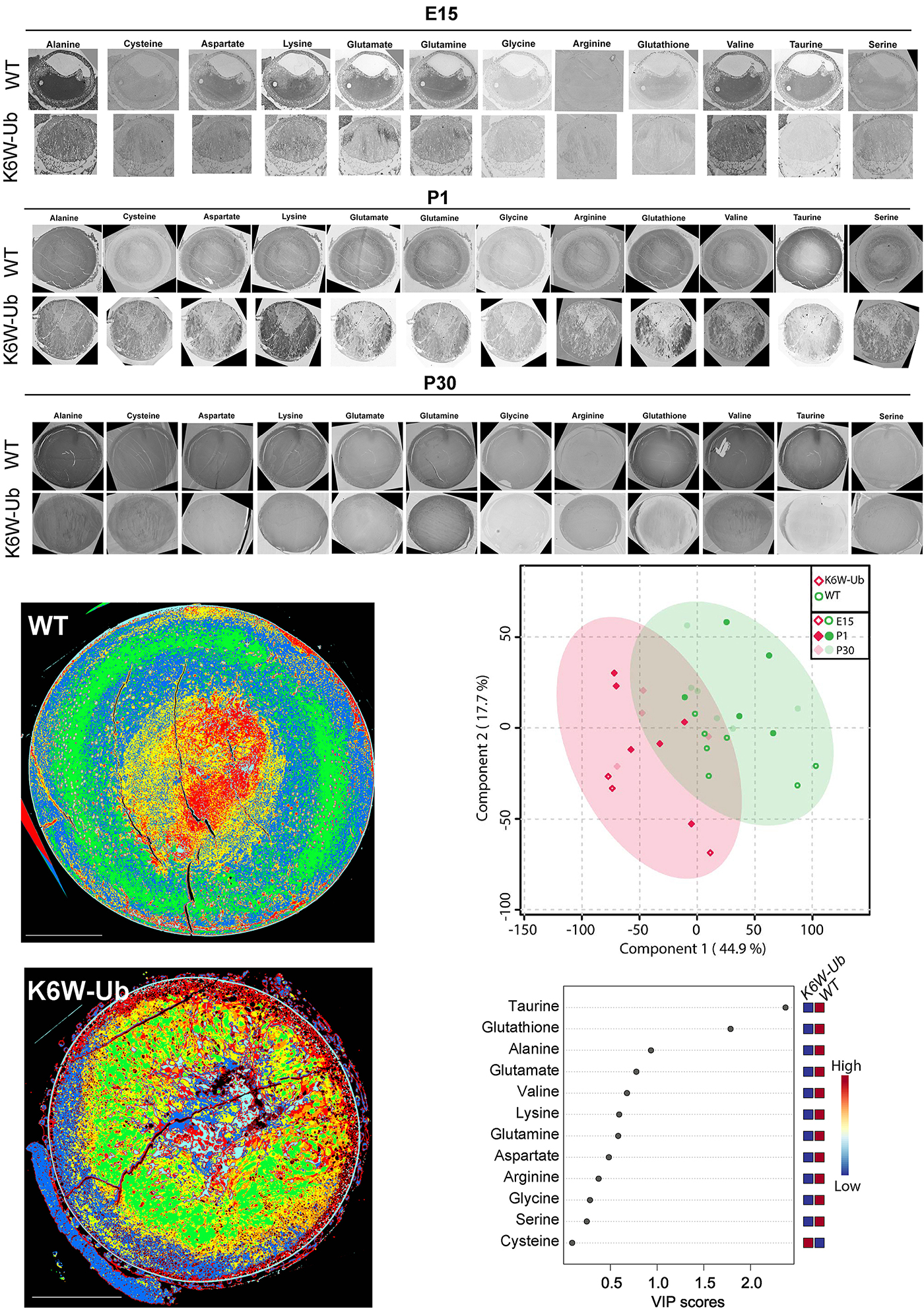
Abstract: The lens proteome undergoes dramatic composition changes during development and maturation. A defective developmental process leads to congenital cataracts that account for about 30% of cases of childhood blindness. Gene mutations are associated with approximately 50% of early-onset forms of lens opacity, with the remainder being of unknown etiology. To gain a better understanding of cataractogenesis, we utilized a transgenic mouse model expressing a mutant ubiquitin protein in the lens (K6W-Ub) that recapitulates most of the early pathological changes seen in human congenital cataracts. We performed mass spectrometry-based tandem-mass-tag quantitative proteomics in E15, P1, and P30 control or K6W-Ub lenses. Our analysis identified targets that are required for early normal differentiation steps and altered in cataractous lenses, particularly metabolic pathways involving glutathione and amino acids. Computational molecular phenotyping revealed that glutathione and taurine were spatially altered in the K6W-Ub cataractous lens. High-performance liquid chromatography revealed that both taurine and the ratio of reduced glutathione to oxidized glutathione, two indicators of redox status, were differentially compromised in lens biology. In sum, our research documents that dynamic proteome changes in a mouse model of congenital cataracts impact redox biology in lens. Our findings shed light on the molecular mechanisms associated with congenital cataracts and point out that unbalanced redox status due to reduced levels of taurine and glutathione, metabolites already linked to age-related cataract, could be a major underlying mechanism behind lens opacities that appear early in life.
Metabolic changes and retinal remodeling in Heterozygous CRX mutant cats (CRXRDY/+)
We have a new manuscript from the lab in Experimental Eye Research, (PubMed link here). Metabolic changes and retinal remodeling in Heterozygous CRX mutant cats (CRXRDY/+). This manuscript is in collaboration with the Simon Petersen-Jones lab out of Michigan State University. Authors are: Laurence M. Occelli, Bryan W. Jones @BWJones, Taylor J. Cervantes, and Simon M. Petersen-Jones. The PDF is here.
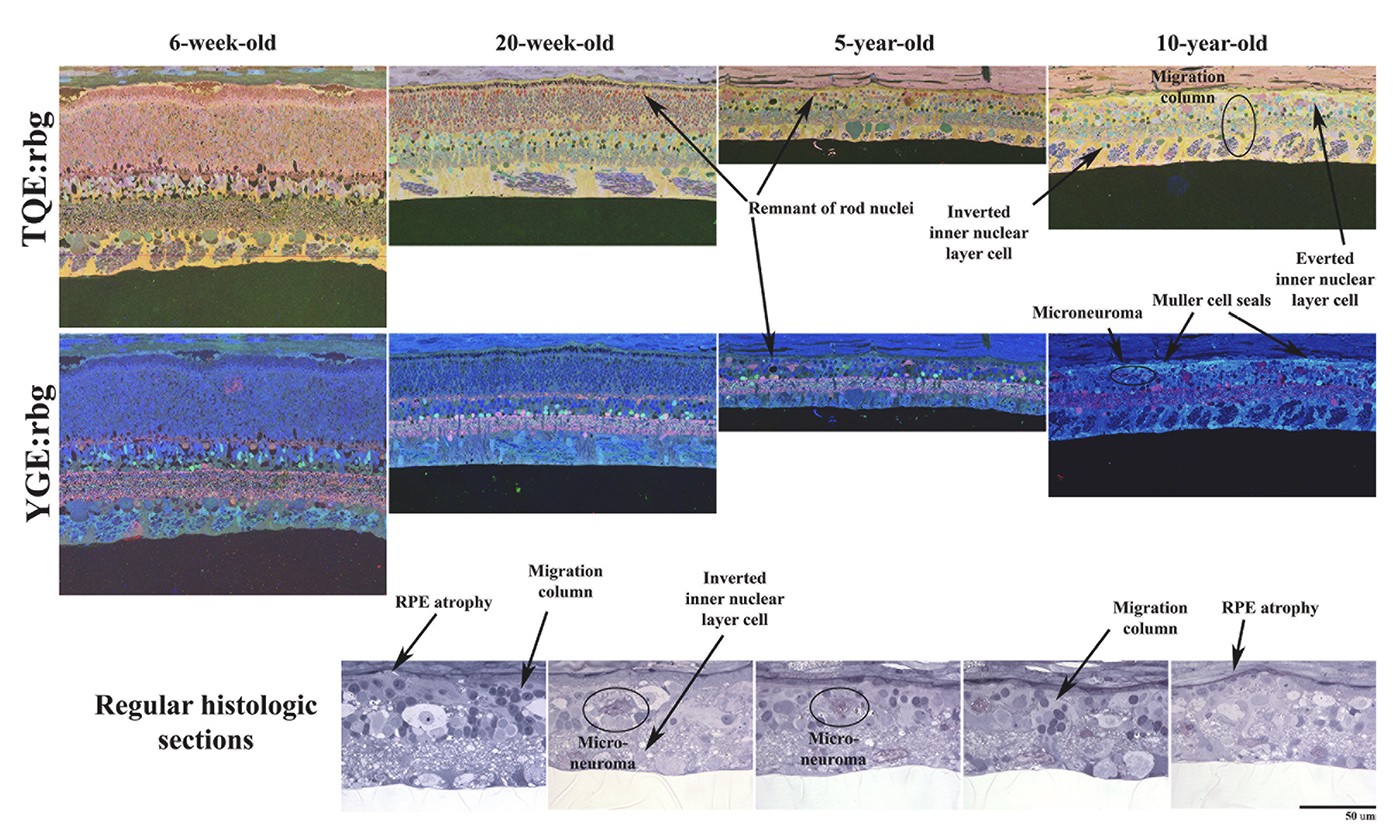
Abstract: CRX is a transcription factor essential for normal photoreceptor development and survival. The CRXRdy cat has a naturally occurring truncating mutation in CRX and is a large animal model for dominant Leber congenital amaurosis. This study investigated retinal remodeling that occurs as photoreceptors degenerate. CRXRdy/+ cats from 6 weeks to 10 years of age were investigated. In vivo structural changes of retinas were analyzed by fundus examination, confocal scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Histologic analyses including immunohistochemistry for computational molecular phenotyping with macromolecules and small molecules. Affected cats had a cone-led photoreceptor degeneration starting in the area centralis. Initially there was preservation of inner retinal cells such as bipolar, amacrine and horizontal cells but with time migration of the deafferented neurons occurred. Early in the process of degeneration glial activation occurs ultimately resulting in formation of a glial seal. With progression the macula-equivalent area centralis developed severe atrophy including loss of retinal pigmentary epithelium. Microneuroma formation occurs in advanced stages as more marked retinal remodeling occurred. This study indicates that retinal degeneration in the CrxRdy/+ cat retina follows the progressive, phased revision of retina that have been previously described for retinal remodeling. These findings suggest that therapy dependent on targeting inner retinal cells may be useful in young adults with preserved inner retinas prior to advanced stages of retinal remodeling and neuronal cell loss.
Impact of Retinal Degeneration on Response of ON and OFF Cone Bipolar Cells to Electrical Stimulation
We have a new manuscript from the lab in IEEE, Impact of Retinal Degeneration on Response of ON and OFF Cone Bipolar Cells to Electrical Stimulation. This manuscript is in collaboration with the Lazzi lab out of USC. The first author, Shayan Farzad, Pragya Kosta, Ege Iseri, Steven T Walston, Jean-Marie C. Bouteiller, Rebecca L. Pfeiffer @BeccaPfeiffer19, Crystal L. Sigulinsky @CSigulinsky, Jia-Hui Yang, Jessica C. Garcia, James R. Anderson, Bryan W. Jones @BWJones, and Gianluca Lazzi. The PDF is here.
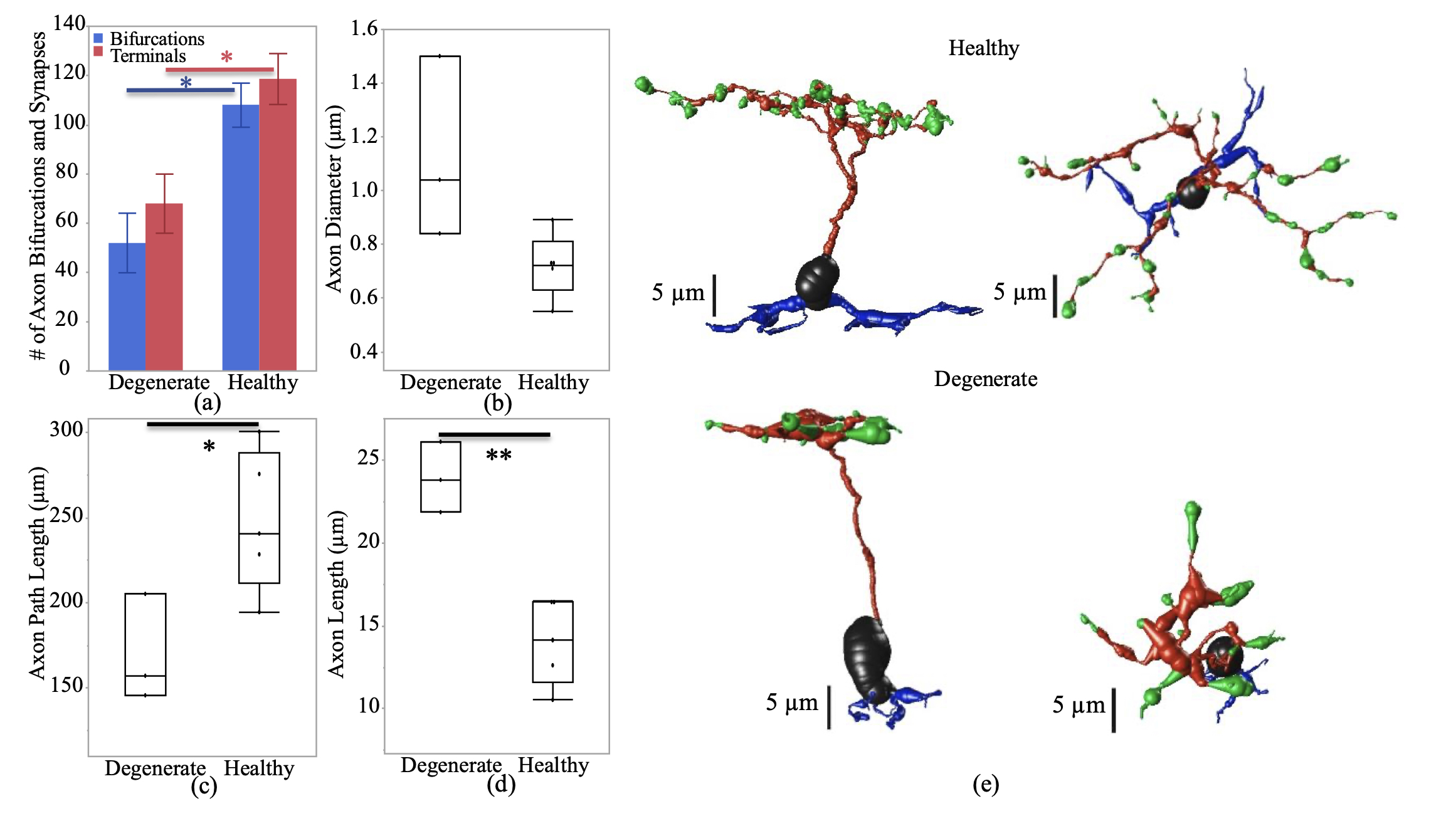
Abstract: In retinal degenerative diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD), the photoreceptors become stressed and start to degenerate in the early stages of the disease. Retinal prosthetic devices have been developed to restore vision in patients by applying electrical stimulation to the surviving retinal cells. However, these devices provide limited visual perception as the therapeutic interventions are generally considered in the later stages of the disease when only inner retinal layer cells are left. A potential treatment option for retinal degenerative diseases in the early stages can be stimulating bipolar cells, which receive presynaptic signals from photoreceptors. In this work, we constructed computational models of healthy and degenerated (both ON and OFF-type) cone bipolar cells (CBCs) with realistic morphologies extracted from connectomes of the healthy and early-stage degenerated rabbit retina. We examined these cells’ membrane potential and axon terminal calcium current differences when subjected to electrical stimulation. In addition, we investigated how differently healthy and degenerated cells behave with respect to various stimulation parameters, including pulse duration and cells’ distance from the stimulating electrode. The results suggested that regardless of the position of the OFF CBCs in the retina model, there is not a significant difference between the membrane potential of healthy and degenerate cells when electrically stimulated. However, the healthy ON CBC axon terminal membrane potential rising time-constant is shorter (0.29 ± 0.03 ms) than the degenerated cells (0.8 ± 0.07 ms). Moreover, the ionic calcium channels at the axon terminals of the cells have a higher concentration and higher current in degenerated cells (32.24 ± 6.12 pA) than the healthy cells (13.64 ± 2.88 pA) independently of the cell’s position.