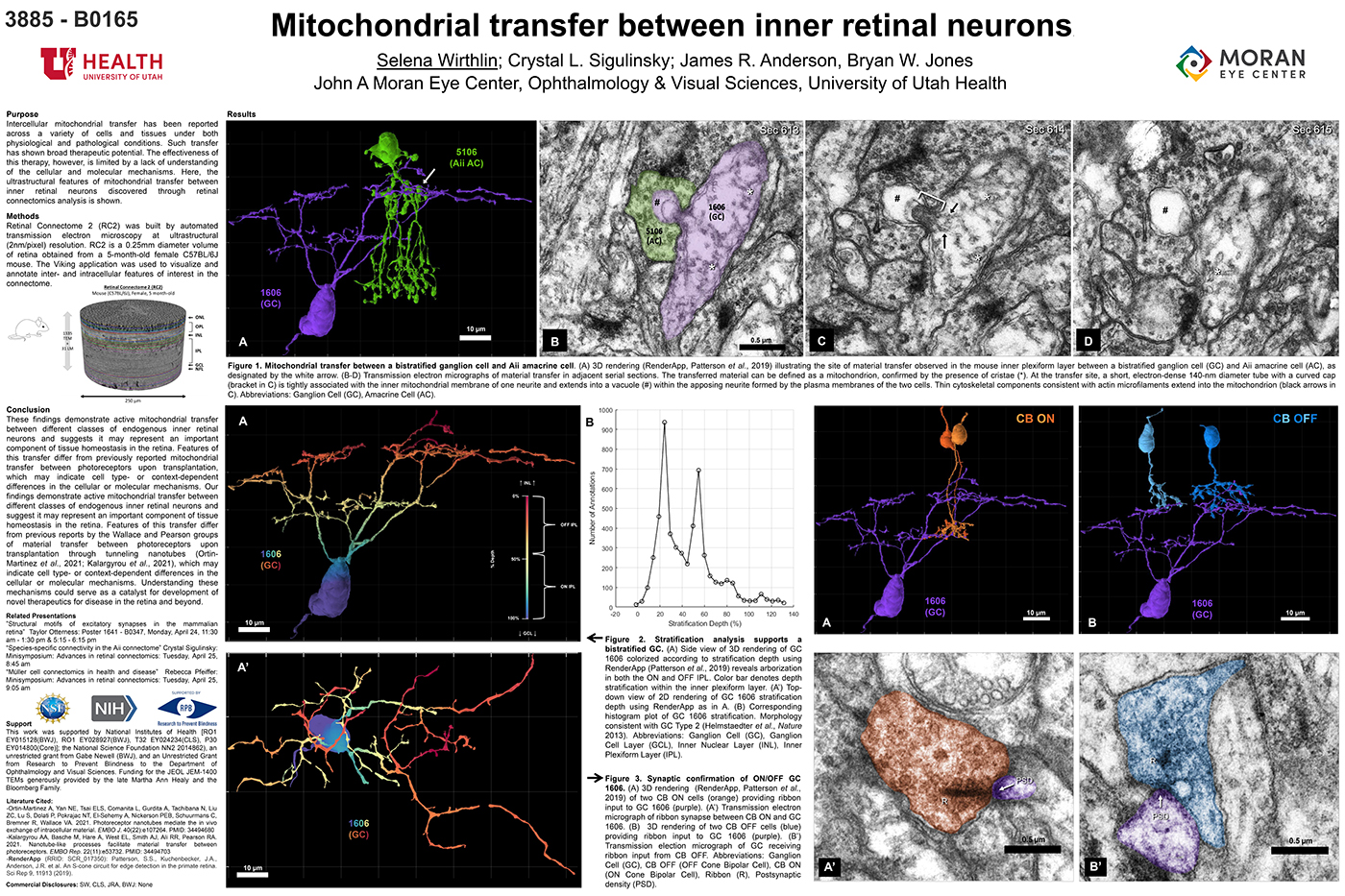
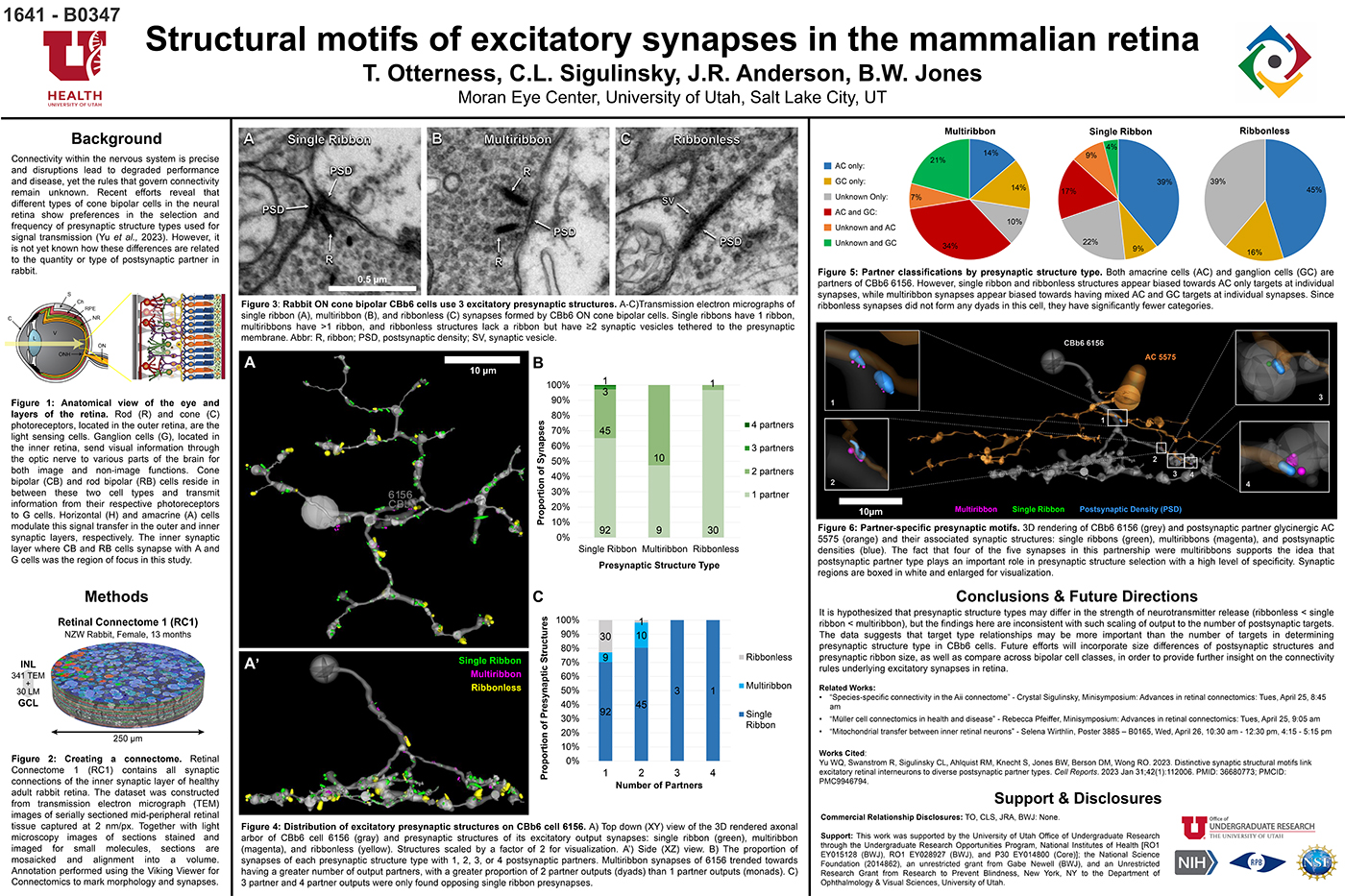
We have a new manuscript from the lab in IEEE, Impact of Retinal Degeneration on Response of ON and OFF Cone Bipolar Cells to Electrical Stimulation. This manuscript is in collaboration with the Lazzi lab out of USC. The first author, Shayan Farzad, Pragya Kosta, Ege Iseri, Steven T Walston, Jean-Marie C. Bouteiller, Rebecca L. Pfeiffer @BeccaPfeiffer19, Crystal L. Sigulinsky @CSigulinsky, Jia-Hui Yang, Jessica C. Garcia, James R. Anderson, Bryan W. Jones @BWJones, and Gianluca Lazzi. The PDF is here.
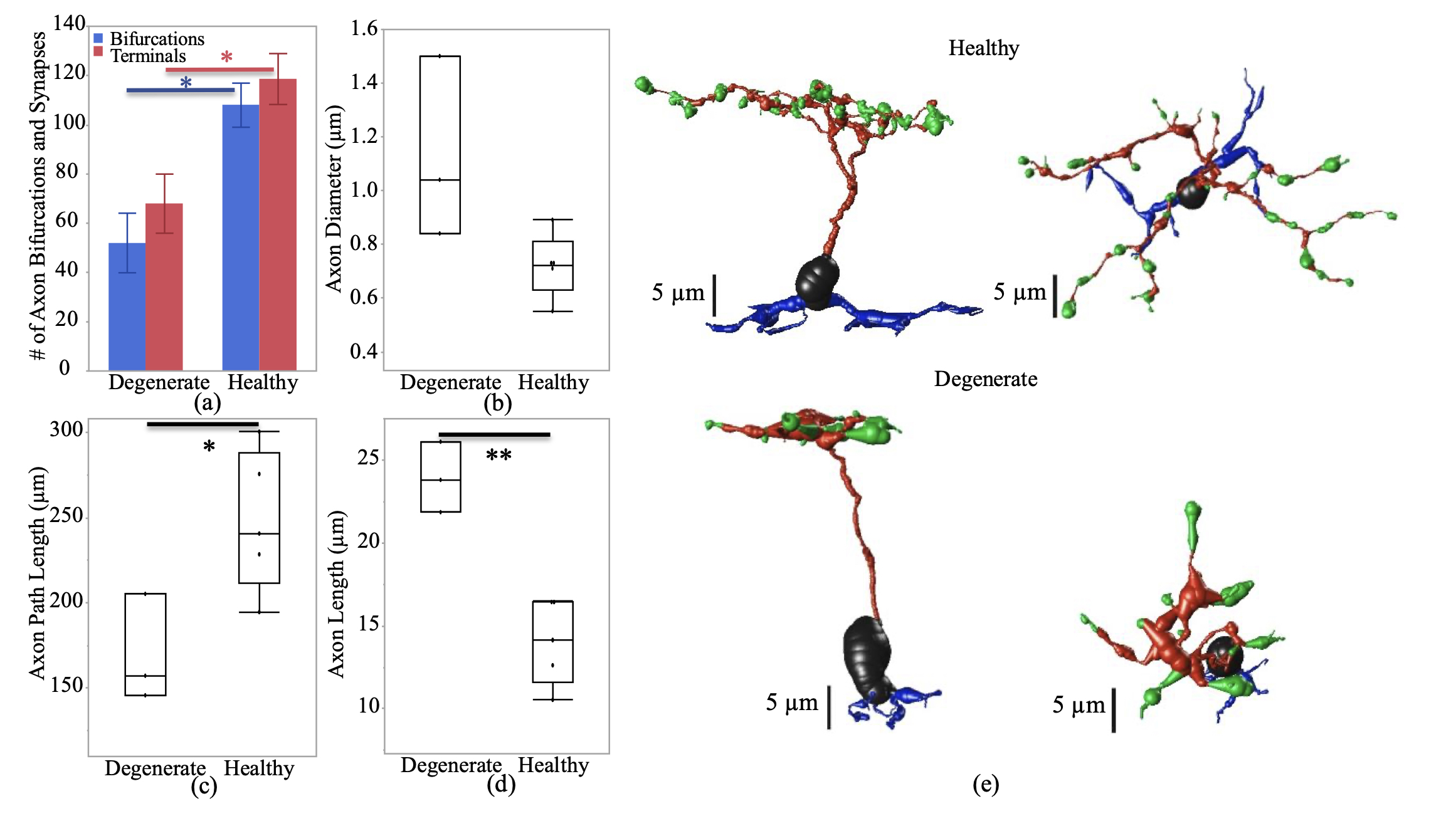
Abstract: In retinal degenerative diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD), the photoreceptors become stressed and start to degenerate in the early stages of the disease. Retinal prosthetic devices have been developed to restore vision in patients by applying electrical stimulation to the surviving retinal cells. However, these devices provide limited visual perception as the therapeutic interventions are generally considered in the later stages of the disease when only inner retinal layer cells are left. A potential treatment option for retinal degenerative diseases in the early stages can be stimulating bipolar cells, which receive presynaptic signals from photoreceptors. In this work, we constructed computational models of healthy and degenerated (both ON and OFF-type) cone bipolar cells (CBCs) with realistic morphologies extracted from connectomes of the healthy and early-stage degenerated rabbit retina. We examined these cells’ membrane potential and axon terminal calcium current differences when subjected to electrical stimulation. In addition, we investigated how differently healthy and degenerated cells behave with respect to various stimulation parameters, including pulse duration and cells’ distance from the stimulating electrode. The results suggested that regardless of the position of the OFF CBCs in the retina model, there is not a significant difference between the membrane potential of healthy and degenerate cells when electrically stimulated. However, the healthy ON CBC axon terminal membrane potential rising time-constant is shorter (0.29 ± 0.03 ms) than the degenerated cells (0.8 ± 0.07 ms). Moreover, the ionic calcium channels at the axon terminals of the cells have a higher concentration and higher current in degenerated cells (32.24 ± 6.12 pA) than the healthy cells (13.64 ± 2.88 pA) independently of the cell’s position.