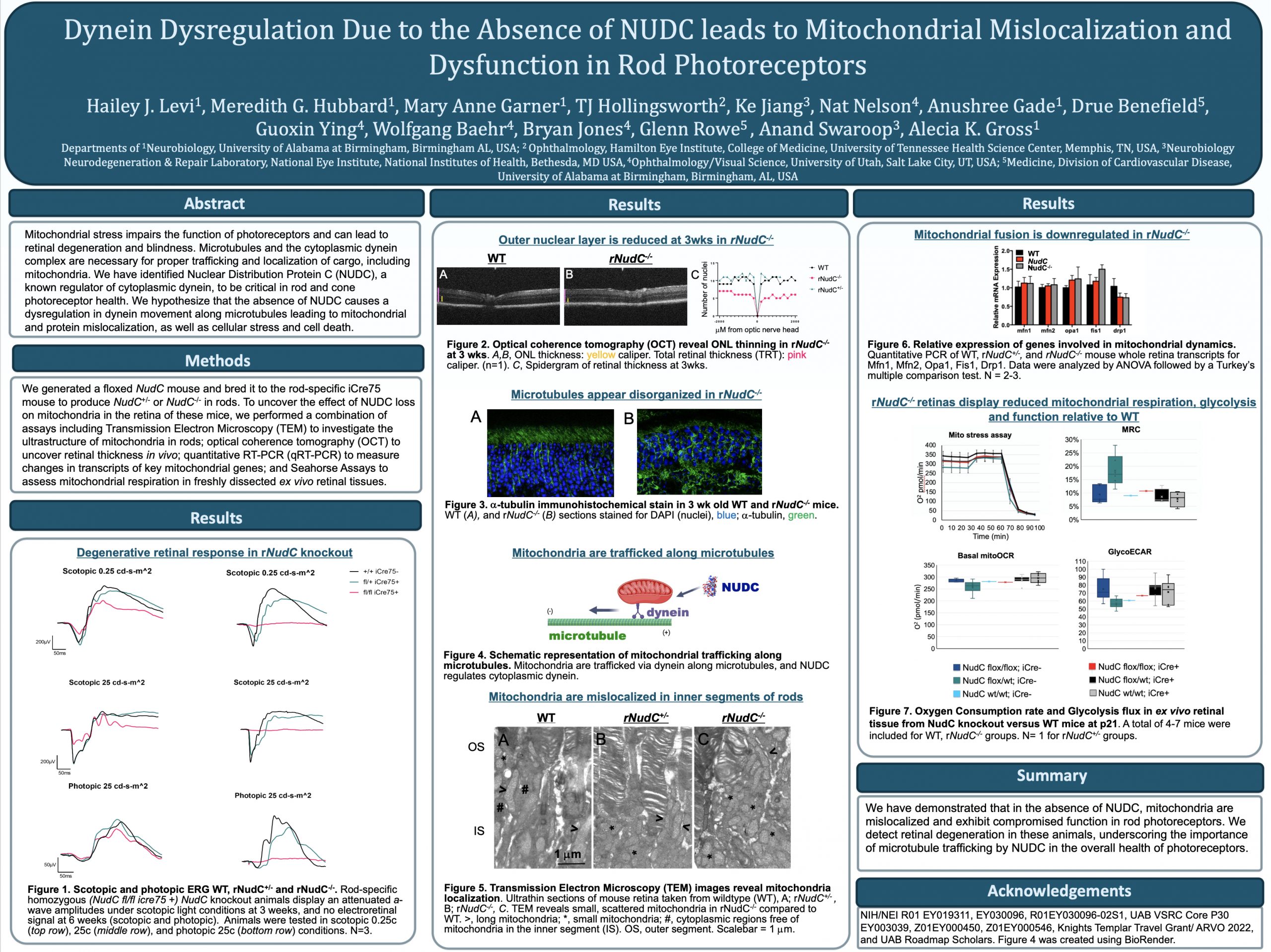
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Hailey Levi @drpepperis100, Meredith Hubbard, Mary Anne Garner, TJ Hollingsworth, Ke Jiang, Nat Nelson, Anushree Gade, Drue Benefield, Guoxin Ying, Wolfgang Baehr, Bryan Jones@BWJones, Anand Swaroop, Glenn Rowe, and Alecia Gross @alecia144g.
Tag Archives: ARVO poster
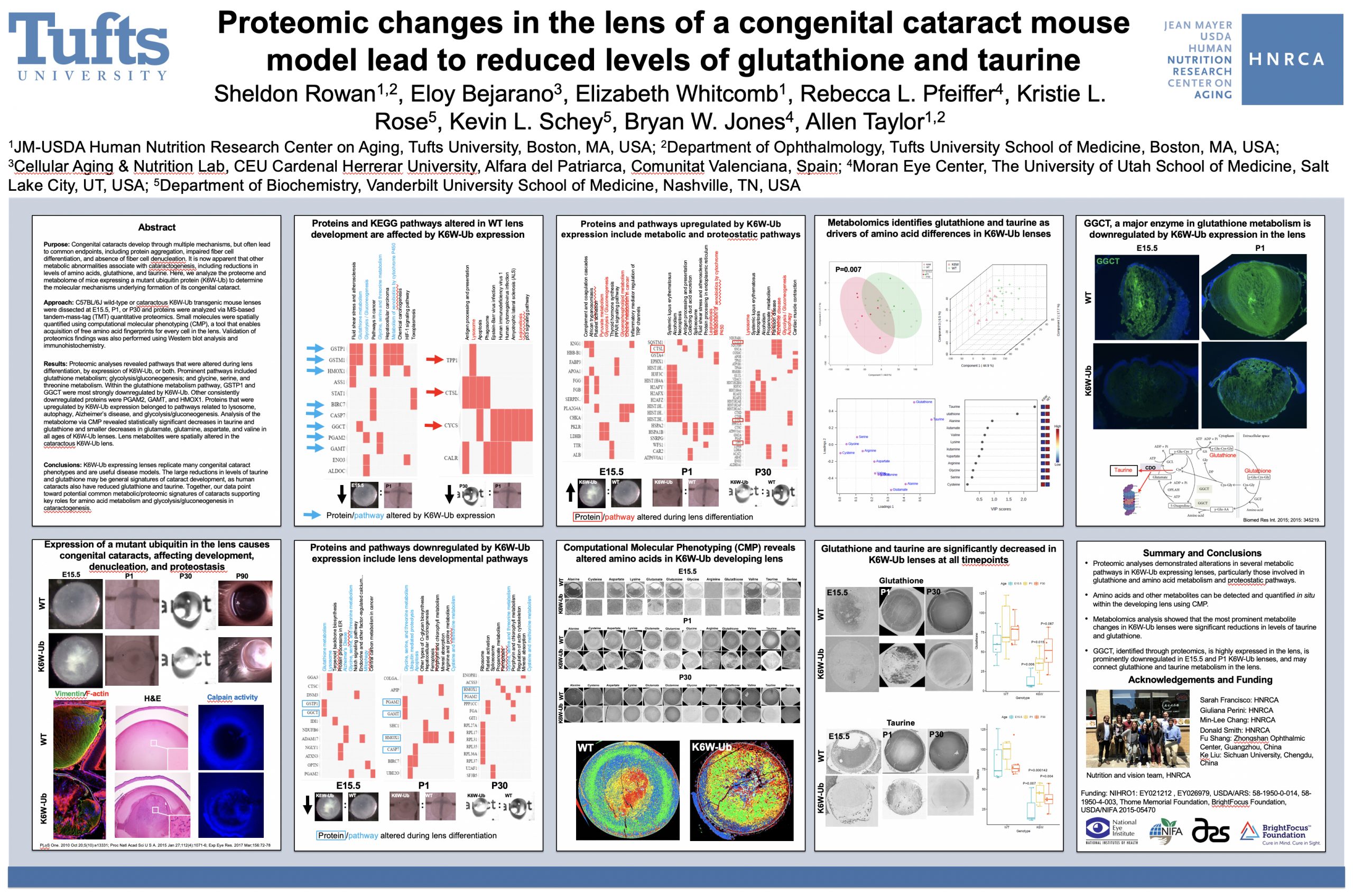
Proteomic changes in the lens of a congenital cataract mouse model lead to reduced levels of glutathione and taurine
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Sheldon Rowan @SheldonRowan, Eloy Bejarano, Elizabeth Whitcomb, Rebecca Pfeiffer @BeccaPfeiffer19, Kristie Rose, Kevin Schey, Bryan Jones @BWJones, Allen Taylor.
Purpose: Congenital cataracts develop through multiple mechanisms, but often lead to common endpoints, including protein aggregation, impaired fiber cell differentiation, and absence of fiber cell denucleation. It is now apparent that other metabolic abnormalities associate with cataractogenesis, including reductions in levels of amino acids, glutathione, and taurine. Here, we analyze the proteome and metabolome of mice expressing a mutant ubiquitin protein (K6W-Ub) to determine the molecular mechanisms underlying formation of its congenital cataract.
Methods: C57BL/6J wild-type or cataractous K6W-Ub transgenic mouse lenses were dissected at E15.5, P1, or P30 and proteins were analyzed via MS-based tandem-mass-tag (TMT) quantitative proteomics. Small molecules were spatially quantified using computational molecular phenotyping (CMP), a tool that enables acquisition of free amino acid fingerprints for every cell in the lens. Validation of proteomics findings was also performed using Western blot analysis and immunohistochemistry.
Results: Proteomic analyses revealed pathways that were altered during lens differentiation, by expression of K6W-Ub, or both. Prominent pathways included glutathione metabolism; glycolysis/gluconeogenesis; and glycine, serine, and threonine metabolism. Within the glutathione metabolism pathway, GSTP1 and GGCT were most strongly downregulated by K6W-Ub. Other consistently downregulated proteins were PGAM2, GAMT, and HMOX1. Proteins that were upregulated by K6W-Ub expression belonged to pathways related to lysosome, autophagy, Alzheimer’s disease, and glycolysis/gluconeogenesis. Analysis of the metabolome via CMP revealed statistically significant decreases in taurine and glutathione and smaller decreases in glutamate, glutamine, aspartate, and valine in all ages of K6W-Ub lenses. Lens metabolites were spatially altered in the cataractous K6W-Ub lens.
Conclusions: K6W-Ub expressing lenses replicate many congenital cataract phenotypes and are useful disease models. The large reductions in levels of taurine and glutathione may be general signatures of cataract development, as human cataracts also have reduced glutathione and taurine. Key roles for amino acid metabolism and glycolysis/gluconeogenesis in cataractogenesis are emerging. Together our data point toward potential common metabolic/proteomic signatures of cataracts.
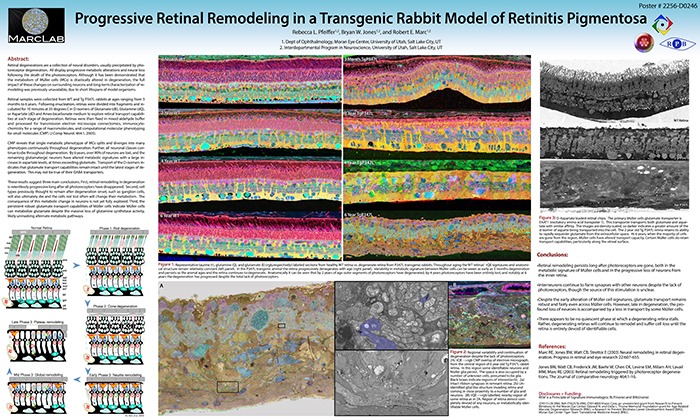
Progressive Retinal Remodeling In A Transgenic Rabbit Model Of Retinitis Pigmentosa
This poster was presented today, May 2th at the 2016 Association for Research in Vision and Opthalmology (ARVO) meetings in Seattle, Washington by Rebecca L. Pfeiffer, Bryan W. Jones, and Robert E. Marc.
Posterboard #: D0246
Abstract Number: 2256 – D0246
Author Block: Rebecca L. Pfeiffer1,2 , Bryan W. Jones1,2 , Robert E. Marc1,2
1 Ophthalmology, University of Utah, Salt Lake City, Utah, United States; 2 Interdepartmental Program in Neuroscience, University of Utah, Salt Lake City, Utah, United States
Purpose:Retinal degenerations are a collection of neural disorders, usually precipitated by photoreceptor degeneration. All display progressive metabolic alterations and neural loss following the death of the photoreceptors. Although it has been demonstrated that the metabolism of Müller cells (MCs) is drastically altered in degeneration, the full impact of these changes on surrounding neurons and long-term characterization of remodeling was previously unavailable, due to short lifespans of model organisms.
Methods:Retinal samples were collected from WT and Tg P347L rabbits at ages ranging from 3 months to 6 years. Following enucleation, retinas were divided into fragments and incubated for 10 minutes at 35 degrees C in D-isomers of Glutamate (dE), Glutamine (dQ), or Aspartate (dD) and Ames-bicarbonate medium to explore retinal transport capabilities at each stage of degeneration. Retinas were then fixed in mixed aldehyde buffer and processed for transmission electron microscope connectomics, immunocytochemistry for a range of macromolecules, and computational molecular phenotyping for small molecules (CMP) (J Comp Neurol. 464:1, 2003).
Results:CMP reveals that single metabolic phenotype of MCs splits and diverges into many phenotypes continuously throughout degeneration. Further, all neuronal classes continue to die throughout degeneration. By 6 years, over 90% of neurons are lost, and the remaining glutamatergic neurons have altered metabolic signatures with a large increase in aspartate levels, at times exceeding glutamate. Transport of the D-isomers indicates that glutamate transport capabilities remain intact until the latest stages of degeneration. This may not be true of their GABA transporters.
Conclusions:These results suggest three main conclusions. First, retinal remodeling in degeneration is relentlessly progressive long after all photoreceptors have disappeared. Second, cell types previously thought to remain after degeneration onset, such as ganglion cells, will also ultimately die and the cells not lost often will change their metabolism. The consequence of this metabolic change in neurons is not yet fully explored. Third, the persistent robust glutamate transport capabilities of Müller cells indicate Müller cells can metabolize glutamate despite the massive loss of glutamine synthetase activity, likely unmasking alternate metabolic pathways.
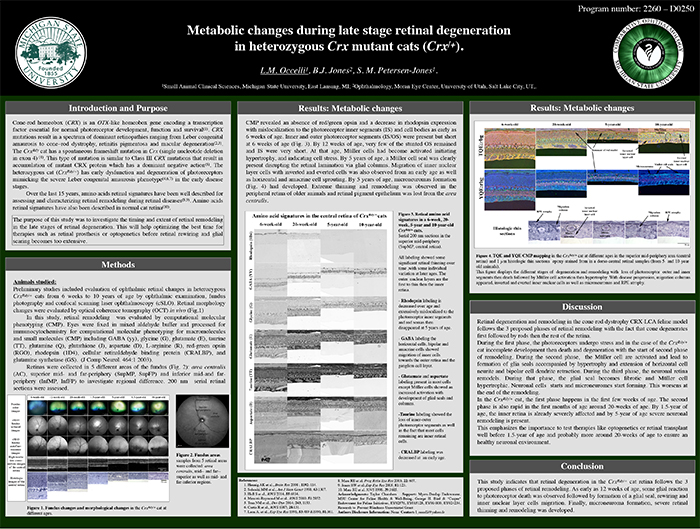
Metabolic Changes During Late Stage Retinal Degeneration In Heterozygous Crx Mutant Cats (CrxRdy/+)
This abstract was presented today, May 2th at the 2016 Association for Research in Vision and Opthalmology (ARVO) meetings in Seattle, Washington by Laurence Occelli, Bryan W. Jones, and Simon M. Petersen-Jones.
Posterboard #: D0250
Abstract Number: 2260 – D0250
Author Block: Laurence M. Occelli1 , Bryan W. Jones2 , Simon M. Petersen-Jones1
1 Small Animal Clinical Sciences, Michigan State University, East Lansing, Michigan, United States; 2 Ophthalmology, Moran Eye Center, University of Utah, Salt Lake City, Utah, United States
Disclosure Block:Laurence M. Occelli, None; Bryan W. Jones, None; Simon M. Petersen-Jones, None
Purpose: CRX is a transcription factor essential for normal photoreceptor development and survival. The CrxRdy cat has a spontaneous mutation in Crx. Early disease stages in heterozygous cats (CrxRdy/+) mimics severe Leber’s congenital amaurosis. This study investigated the timing and extent of retinal remodeling in the late stages of retinal degeneration. This will help optimizing the best time for therapies such as retinal prosthesis or optogenetics before retinal rewiring and glial scar become too extensive.
Methods: CrxRdy/+ cats from 6 weeks to 10 years of age were investigated. Eyes were fixed in mixed aldehyde buffer and processed for immunocytochemistry for computational molecular phenotyping for macromolecules and small molecules (CMP) including GABA, glycine, glutamate, taurine, glutamine, aspartate, rhodopsin and red green opsin (J Comp Neurol. 464:1 2003). Samples from 5 retinal areas were collected: area centralis, mid- and far-superior as well as mid- and far-inferior regions.
Results: CMP revealed an absence of red green opsin and a decrease in rhodopsin expression with mislocalization to the photoreceptor inner segments (IS) and cell bodies as early as 6 weeks of age. Inner and outer photoreceptor segments (IS/OS) were present but short at 6 weeks of age. By 12 weeks of age, very few of the stunted OS remained and IS were very short. At that age, Müller cells had become activated initiating hypertrophy, and indicating cell stress. By 5 years of age, a Müller cell seal was clearly present disrupting the retinal lamination via glial columns. Migration of inner nuclear layer cells with inverted and everted cells was also observed from an early age as well as horizontal and amacrine cell sprouting. By 5 years of age, microneuromas formations had developed (Fig.1). Extreme thinning and remodeling was observed in the peripheral retina of older animals and retinal pigment epithelium was lost from the area centralis.
Conclusions: This study indicates that retinal degeneration in the CrxRdy/+ cat retina follows the 3 proposed phases of retinal remodeling. As early as 12 weeks of age, some glial reaction to photoreceptor death was observed followed by formation of a glial seal, rewiring and inner nuclear layer cells migration. Finally, microneuroma formation, severe retinal thinning and remodeling was developed.