We are pleased to reveal that Dr. Bryan William Jones has been selected for an RPB Stein Innovation Award from Research to Prevent Blindness. This particular project is something that we’ve been scheming for a while and leverages an approach to comparative anatomy to study the ground squirrel retina. The unique thing about the 12-lined ground squirrel retina is that the photoreceptors of this organism degenerate when it hibernates. The outer segments of the photoreceptors degenerate and the synapses that connect them to the first synapse of the visual system dissolves in much the same way as when the retina degenerates in human diseases like retinitis pigmentosa, and age-related macular degeneration. The trick is: When the 13-lined ground squirrel comes out of hibernation, their retinas regenerate and their synapses reconnect giving us an incredible opportunity to explore plasticity in their nervous systems.
Tag Archives: retina
Bryan William Jones TEDx Berlin Talk: How Your Vision Determines Your Reality
In February, lab PI Bryan William Jones traveled to Berlin to give a talk for TEDx Berlin. Now the full talk from his presentation at TEDx Berlin has been posted.
NUDC Is Critical For Rod Photoreceptor Function, Maintenance, And Survival
We have a new manuscript in collaboration with the Gross Lab out of UAB in The FASEB Journal, (PubMed link here): NUDC is critical for rod photoreceptor function, maintenance, and survival. This manuscript is in collaboration with. Authors are: Mary Anne Garner, Meredith G. Hubbard, Evan R. Boitet, Seth T. Hubbard, Anushree Gade, Guoxin Ying, Bryan W. Jones, Wolfgang Baehr, Alecia K. Gross. The PDF is here.
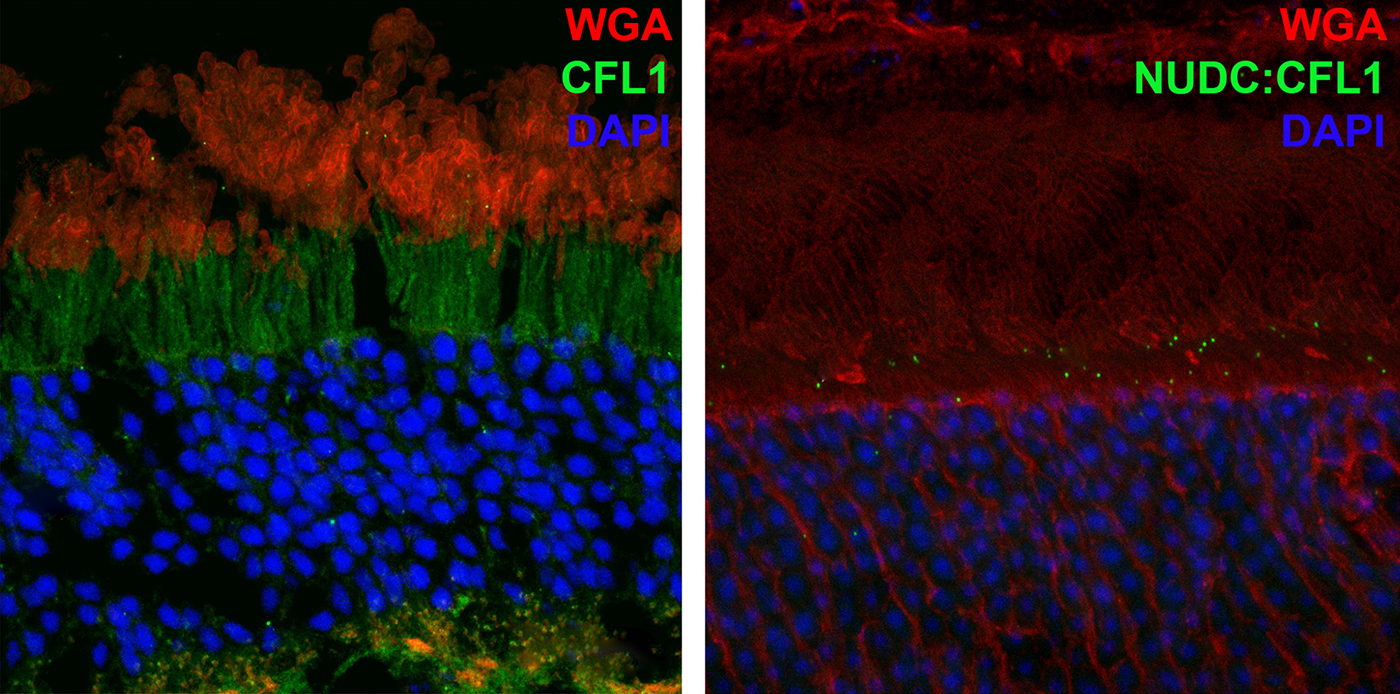
Abstract: NUDC (nuclear distribution protein C) is a mitotic protein involved in nuclear migration and cytokinesis across species. Considered a cytoplasmic dynein (henceforth dynein) cofactor, NUDC was shown to associate with the dynein motor complex during neuronal migration. NUDC is also expressed in postmitotic vertebrate rod photoreceptors where its function is unknown. Here, we examined the role of NUDC in postmitotic rod photoreceptors by studying the consequences of a conditional NUDC knockout in mouse rods (rNudC−/−). Loss of NUDC in rods led to complete photoreceptor cell death at 6 weeks of age. By 3 weeks of age, rNudC−/− function was diminished, and rhodopsin and mitochondria were mislocalized, consistent with dynein inhibition. Levels of outer segment proteins were reduced, but LIS1 (lissencephaly protein 1), a well-characterized dynein cofactor, was unaffected. Transmission electron microscopy revealed ultrastructural defects within the rods of rNudC−/− by 3 weeks of age. We investigated whether NUDC interacts with the actin modulator cofilin 1 (CFL1) and found that in rods, CFL1 is localized in close proximity to NUDC. In addition to its potential role in dynein trafficking within rods, loss of NUDC also resulted in increased levels of phosphorylated CFL1 (pCFL1), which would purportedly prevent depolymerization of actin. The absence of NUDC also induced an inflammatory response in Müller glia and microglia across the neural retina by 3 weeks of age. Taken together, our data illustrate the critical role of NUDC in actin cytoskeletal maintenance and dynein-mediated protein trafficking in a postmitotic rod photoreceptor.
UCI Center For Translational Vision Research Talk on Retinal Connectomics and Pathoconnectomics
Metabolic changes and retinal remodeling in Heterozygous CRX mutant cats (CRXRDY/+)
We have a new manuscript from the lab in Experimental Eye Research, (PubMed link here). Metabolic changes and retinal remodeling in Heterozygous CRX mutant cats (CRXRDY/+). This manuscript is in collaboration with the Simon Petersen-Jones lab out of Michigan State University. Authors are: Laurence M. Occelli, Bryan W. Jones @BWJones, Taylor J. Cervantes, and Simon M. Petersen-Jones. The PDF is here.
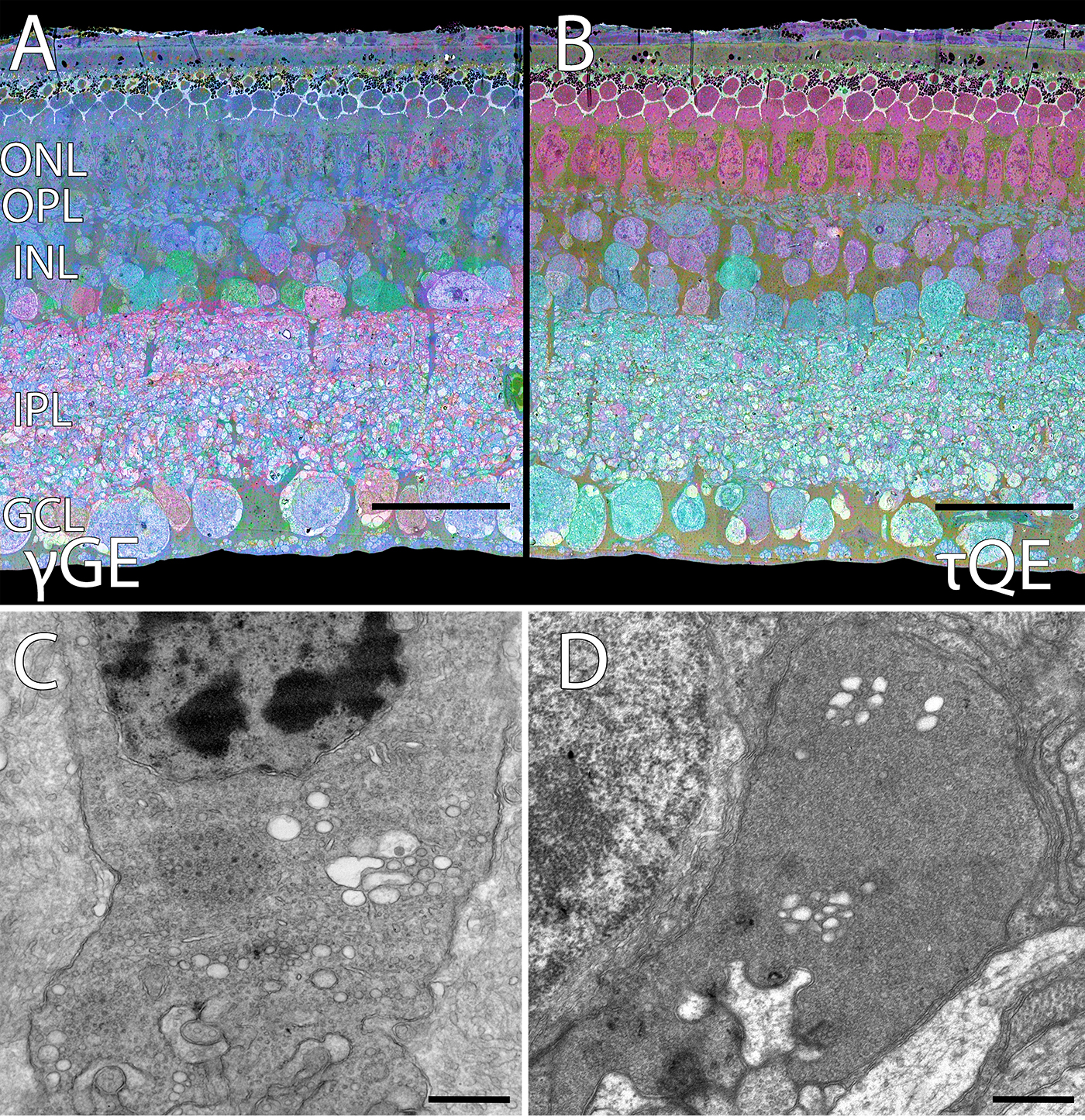
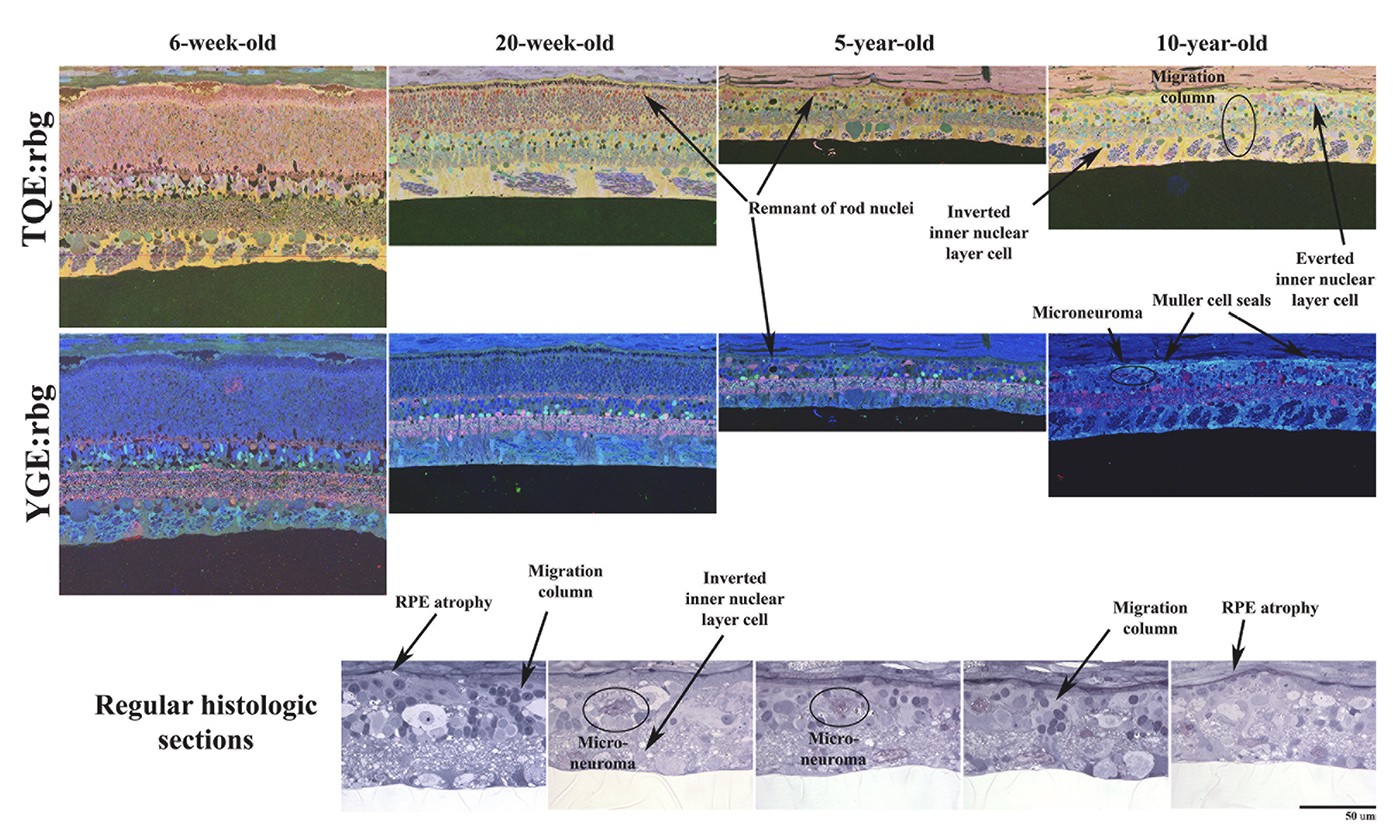
Abstract: CRX is a transcription factor essential for normal photoreceptor development and survival. The CRXRdy cat has a naturally occurring truncating mutation in CRX and is a large animal model for dominant Leber congenital amaurosis. This study investigated retinal remodeling that occurs as photoreceptors degenerate. CRXRdy/+ cats from 6 weeks to 10 years of age were investigated. In vivo structural changes of retinas were analyzed by fundus examination, confocal scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Histologic analyses including immunohistochemistry for computational molecular phenotyping with macromolecules and small molecules. Affected cats had a cone-led photoreceptor degeneration starting in the area centralis. Initially there was preservation of inner retinal cells such as bipolar, amacrine and horizontal cells but with time migration of the deafferented neurons occurred. Early in the process of degeneration glial activation occurs ultimately resulting in formation of a glial seal. With progression the macula-equivalent area centralis developed severe atrophy including loss of retinal pigmentary epithelium. Microneuroma formation occurs in advanced stages as more marked retinal remodeling occurred. This study indicates that retinal degeneration in the CrxRdy/+ cat retina follows the progressive, phased revision of retina that have been previously described for retinal remodeling. These findings suggest that therapy dependent on targeting inner retinal cells may be useful in young adults with preserved inner retinas prior to advanced stages of retinal remodeling and neuronal cell loss.
Impact of Retinal Degeneration on Response of ON and OFF Cone Bipolar Cells to Electrical Stimulation
We have a new manuscript from the lab in IEEE, Impact of Retinal Degeneration on Response of ON and OFF Cone Bipolar Cells to Electrical Stimulation. This manuscript is in collaboration with the Lazzi lab out of USC. The first author, Shayan Farzad, Pragya Kosta, Ege Iseri, Steven T Walston, Jean-Marie C. Bouteiller, Rebecca L. Pfeiffer @BeccaPfeiffer19, Crystal L. Sigulinsky @CSigulinsky, Jia-Hui Yang, Jessica C. Garcia, James R. Anderson, Bryan W. Jones @BWJones, and Gianluca Lazzi. The PDF is here.
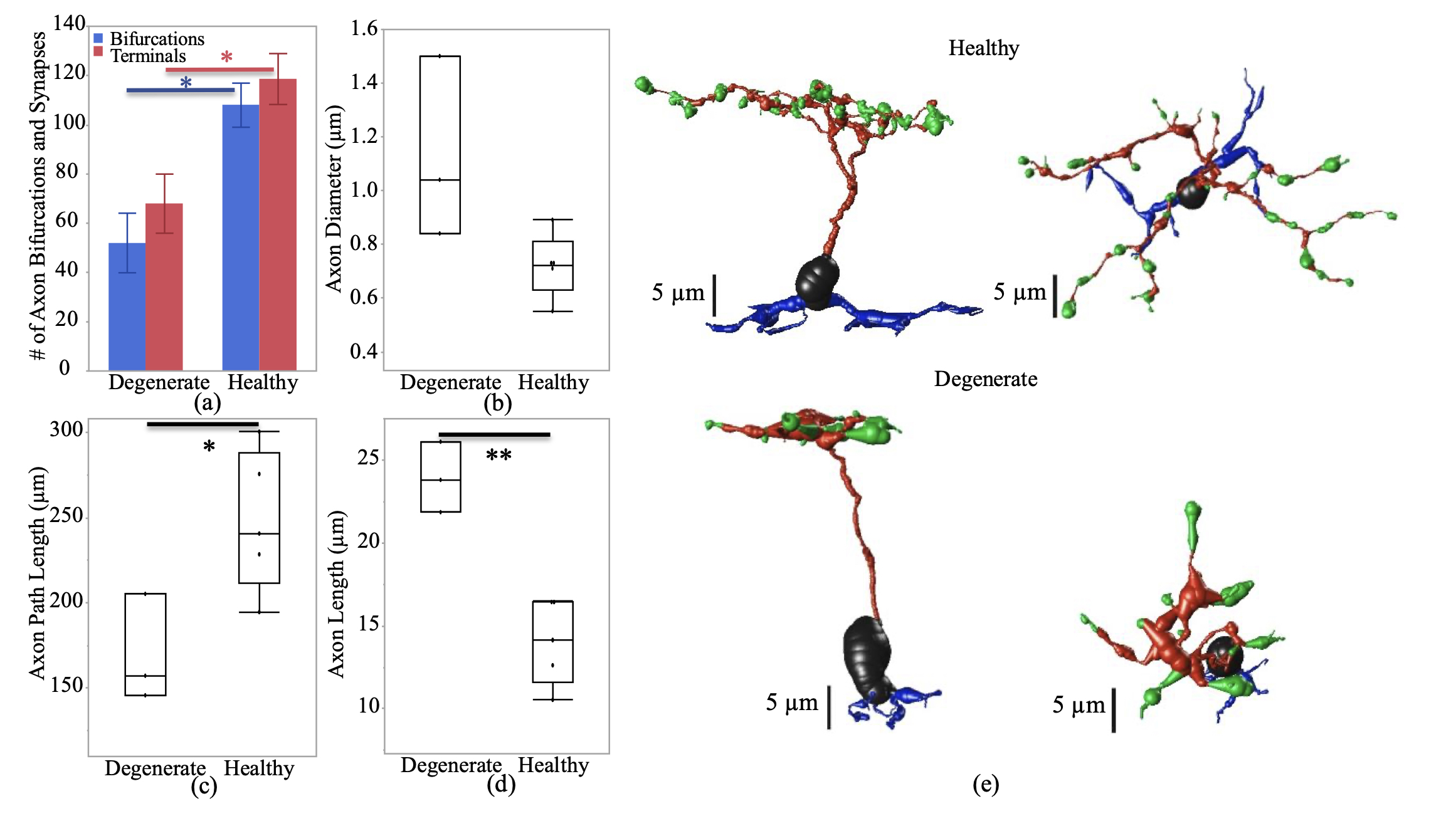
Abstract: In retinal degenerative diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD), the photoreceptors become stressed and start to degenerate in the early stages of the disease. Retinal prosthetic devices have been developed to restore vision in patients by applying electrical stimulation to the surviving retinal cells. However, these devices provide limited visual perception as the therapeutic interventions are generally considered in the later stages of the disease when only inner retinal layer cells are left. A potential treatment option for retinal degenerative diseases in the early stages can be stimulating bipolar cells, which receive presynaptic signals from photoreceptors. In this work, we constructed computational models of healthy and degenerated (both ON and OFF-type) cone bipolar cells (CBCs) with realistic morphologies extracted from connectomes of the healthy and early-stage degenerated rabbit retina. We examined these cells’ membrane potential and axon terminal calcium current differences when subjected to electrical stimulation. In addition, we investigated how differently healthy and degenerated cells behave with respect to various stimulation parameters, including pulse duration and cells’ distance from the stimulating electrode. The results suggested that regardless of the position of the OFF CBCs in the retina model, there is not a significant difference between the membrane potential of healthy and degenerate cells when electrically stimulated. However, the healthy ON CBC axon terminal membrane potential rising time-constant is shorter (0.29 ± 0.03 ms) than the degenerated cells (0.8 ± 0.07 ms). Moreover, the ionic calcium channels at the axon terminals of the cells have a higher concentration and higher current in degenerated cells (32.24 ± 6.12 pA) than the healthy cells (13.64 ± 2.88 pA) independently of the cell’s position.
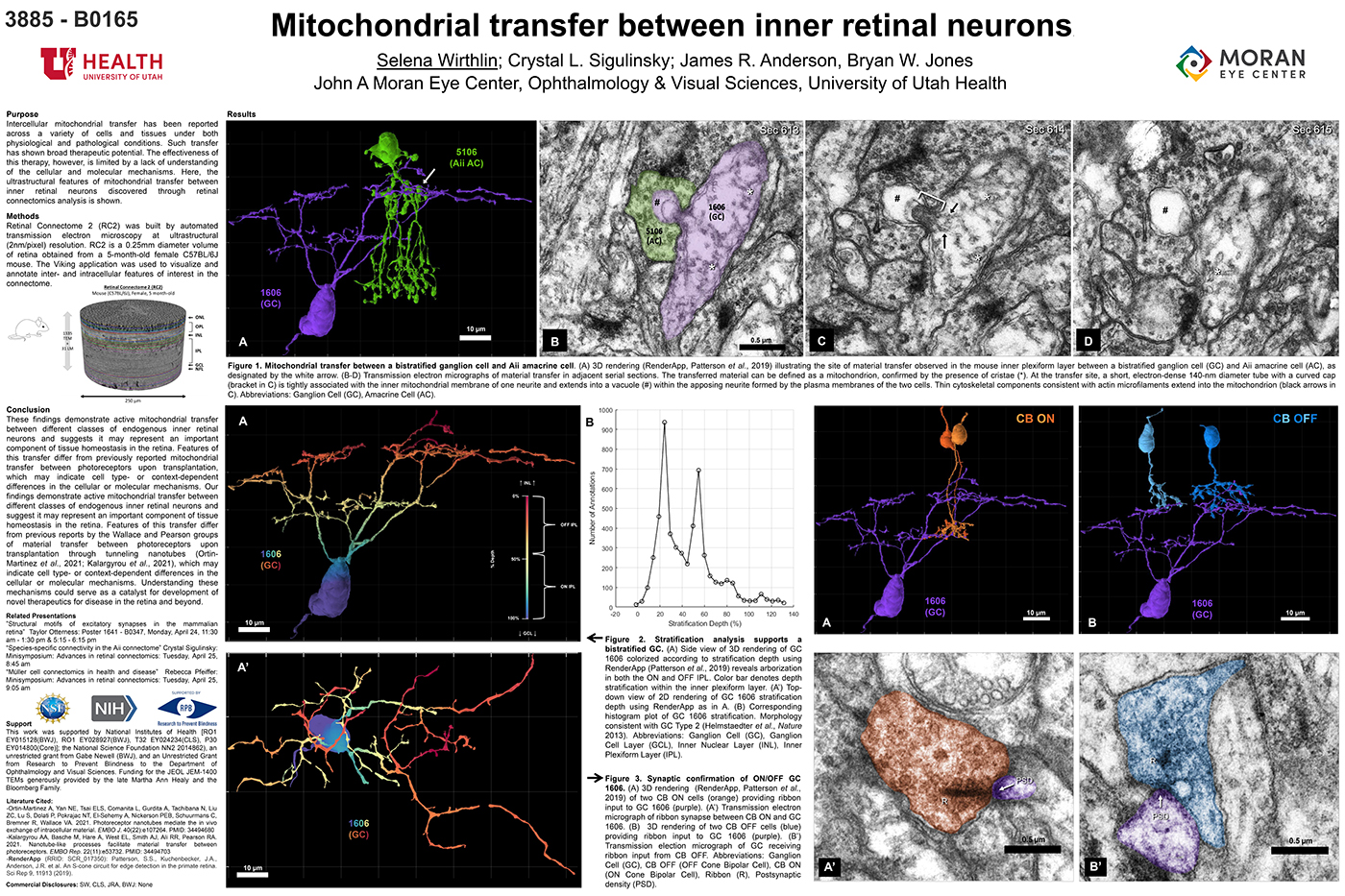
Mitochondrial Transfer Between Inner Retinal Neurons
This abstract was presented today, April 26th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Selena Wirthlin, Crystal Sigulinsky, James Anderson, and Bryan William Jones.
Full resolution version here.
Purpose
Intercellular mitochondrial transfer has been reported across a variety of cells and tissues under both physiological and pathological conditions. Such transfer has shown broad therapeutic potential. The effectiveness of this therapy, however, is limited by a lack of understanding of the cellular and molecular mechanisms. Here, the ultrastructural features of mitochondrial transfer between inner retinal neurons discovered through retinal connectomics analysis is shown.
Methods
Retinal Connectome 2 (RC2) was built by automated transmission electron microscopy at ultrastructural (2nm/pixel) resolution. RC2 is a 0.25mm diameter volume of retina obtained from a 5-month-old female C57BL/6J mouse. The Viking application was used to visualize and annotate inter- and intracellular features of interest in the connectome.
Results
Exploration of RC2 revealed material transfer between apposing neural processes within the OFF subliminal of the inner plexiform layer. The transferred material can be defined as a mitochondria, confirmed by the presence of crustae. At the transfer site, a short, electron-dense 140-nm diameter tube with a curved cap tightly associated with the inner mitochondrial membrane of one neuritis extends into a vacuole within the apposing neuritis formed by the plasma membranes of the two cells. Thin cytoskeletal components consistent with actin microfilaments extend into the mitochondrion. Morphology and synaptology of the acceptor cell confirm it is an Aii amacrine cell, while preliminary findings suggest the donor cell is a type of ON/OFF ganglion cell.
Conclusions
These findings demonstrate active mitochondrial transfer between different classes of endogenous inner retinal neurons and suggests it may represent an important component of tissue homeostasis in the retina. Features of this transfer differ from previously reported mitochondrial transfer between photoreceptors upon transplantation, which may indicate cell type- or context-dependent differences in the cellular or molecular mechanisms. Our findings demonstrate active mitochondrial transfer between different classes of endogenous inner retinal neurons and suggest it may represent an important component of tissue homeostasis in the retina. Features of this transfer differ from previous reports by the Wallace and Pearson groups of material transfer between photoreceptors upon transplantation through tunneling nanotubes (Ortin- Martinez et al., 2021; Kalargyrou et al., 2021), which may indicate cell type- or context-dependent differences in the cellular or molecular mechanisms. Understanding these mechanisms could serve as a catalyst for development of novel therapeutics for disease in the retina and beyond.
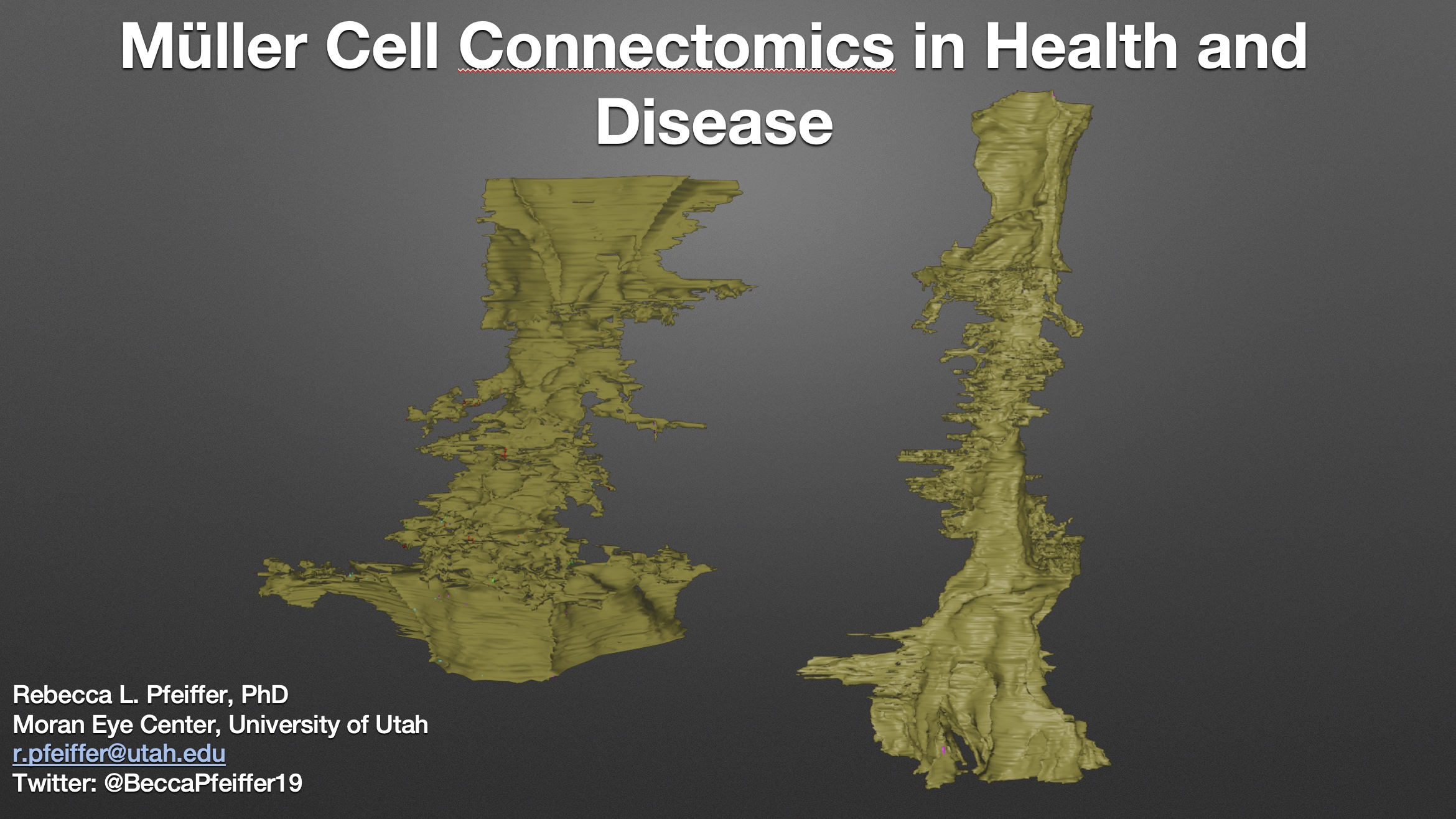
Müller Cell Connectomics In Health And Disease
This talk was presented today, April 25th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Rebecca Pfeiffer as part of an ARVO Minisymposium Bryan William Jones organized.
Abstract: Muller cells are a critical component of retinal function and rapidly change metabolically and morphologically in retinal disease. Of Muller cell functions, many require close physical relationships between the Muller cell and the synapses of the neurons they support. Despite this required neuro-glial relationship, little is known about the direct contacts between Muller cells and synapses in healthy or diseased retinas. In order to address this, I use a connectomics/pathoconnectomics approach to reconstruct Muller cells and their neighboring synapses. The retinas evaluated are from a healthy rabbit, retinal connectome 1 (RC1), and from the P347L rabbit model of retinitis pigmentosa, retinal pathoconnectome 1 (RPC1). Preliminary data demonstrate an increase in endfoot entanglement in RPC1 when compared with RC1, and direct synaptic contact analysis of both connectomes is ongoing.
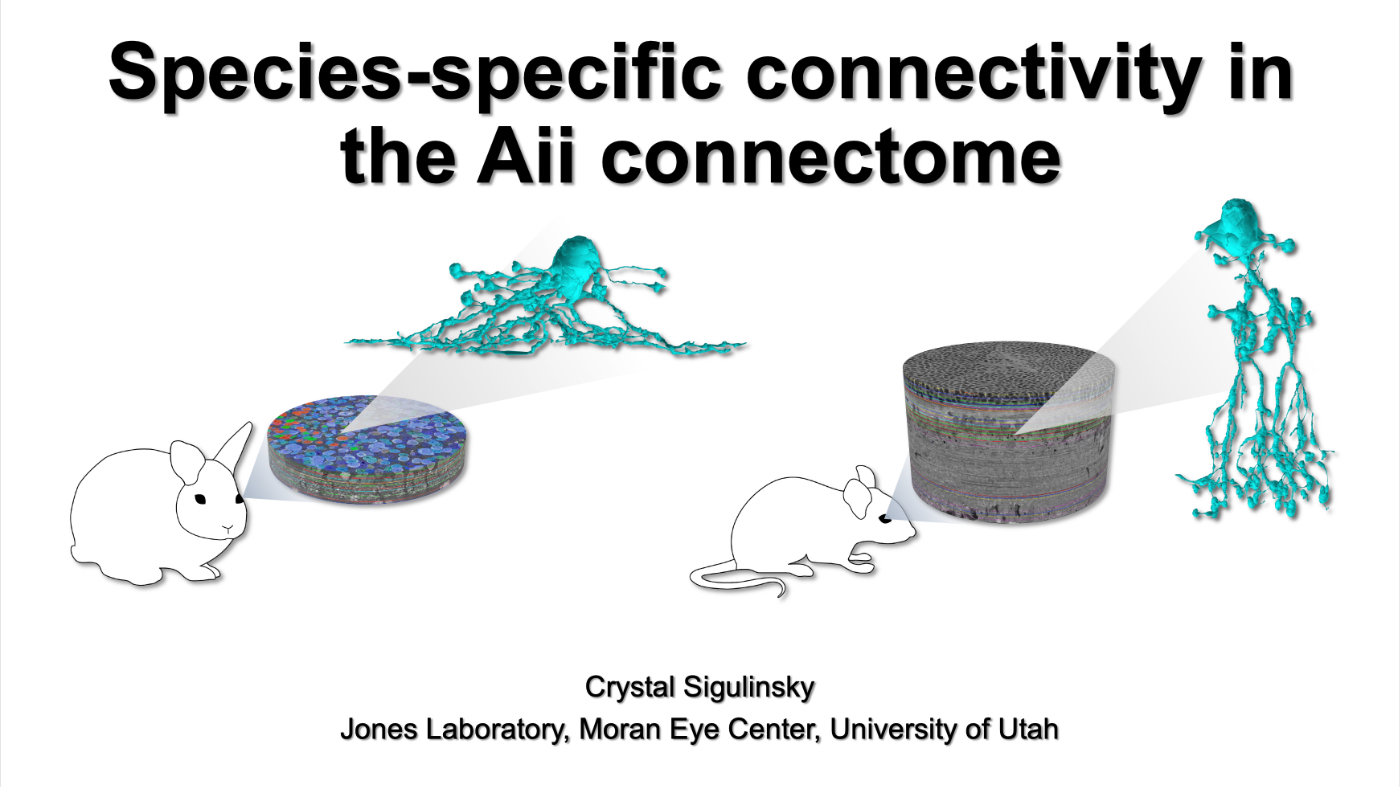
Species-Specific Connectivity In The Aii Connectome
This talk was presented today, April 25th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Crystal Sigulinsky as part of an ARVO Minisymposium organized by Bryan William Jones.
Abstract: Biomedical research relies heavily on animal models to study human disease and develop therapeutics. Understanding the architectural diversity in neural networks between humans and these model species is essential for choosing a relevant study model and interpreting conflicting results. Using comparative connectomics, we sought to map and compare the local neural network architecture of rabbit and mouse retinal Aii amacrine cells. This specialized narrow-field, multistratified, glycinergic interneuron has critical feedforward and feedback roles in both the photopic and scotopic retinal networks spanning the ON and OFF pathways, making it an ideal candidate for investigating species-specific differences in retinal networks. High-resolution, serial-section transmission electron microscopy (TEM) volumes of rabbit (RC1: female, 13- month, Dutch Belted) and mouse (RC2: female, 5-month, C57BL/6J) retinal tissue provided spatially-registered synaptic maps of Aii connectivity at directly comparable resolution and completeness. These reveal that despite species-specific morphologies, gross synaptology and compartmentalization appear conserved. Yet, rabbit and mouse Aii cells diverge in the weighting of their partnerships, most notably in their coupling profiles. Opposing biases in gap junction partnerships and their respective sizing rules indicate a greater relative output by mouse Aii cells to ON pathways than in rabbit. However, a unique topological conformation for a subset of conventional presynapses formed by Aii cell lobular dendrites with species-specific features and prevalence may influence signal output to specific partner classes within the OFF pathway and either nullify or exacerbate this difference in ON/OFF output. Additionally, rabbit Aii cells in RC1 showed greater Aii-Aii coupling than in mouse, which may suggest greater signal-to-noise compensation. Lastly, preliminary data suggest mouse Aii cells receive greater excitatory, but not inhibitory input/feedback from the OFF pathway than in rabbit. Together these data indicate that precise neural circuit architectures diverge between species and require detailed, comprehensive mapping to begin to dissect potential influence on signal flow.
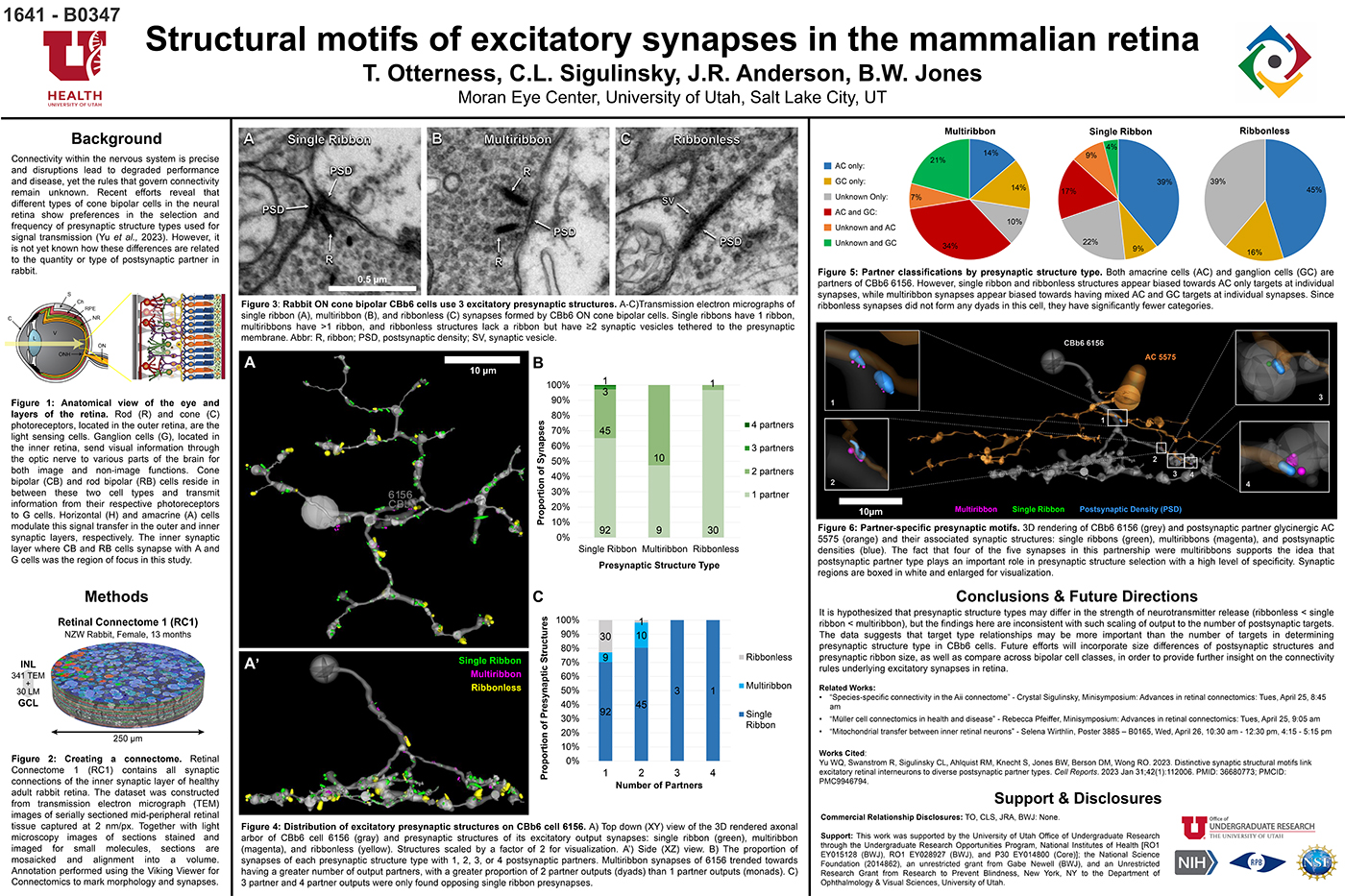
Structural Motifs Of Excitatory Synapses In The Mammalian Retina
This abstract was presented today, April 24th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Taylor Otterness, Crystal Sigulinsky, James Anderson, and Bryan William Jones.
Full resolution version here.
Purpose
Connectivity within the nervous system is precise and disruptions lead to degraded performance and disease, yet the rules that govern connectivity remain unknown. Recent efforts reveal that different types of cone bipolar cells in the neural retina show preferences in the selection and frequency of presynaptic structure types used for signal transmission. However, it is not yet known how these differences are related to the quantity or type of postsynaptic partner. We used Retinal Connectome 1 (RC1) to analyze the synaptic output of rabbit CBb6 cells, a type of ON cone bipolar cell that forms excitatory synapses via diverse presynaptic structure types, to identify patterns in how these cells interact with their postsynaptic partners.
Methods
RC1 is a 0.25 mm diameter volume sampled from mid-peripheral retina of a 13 month old female Dutch-Belted rabbit, serially sectioned at 70 nm, and imaged at ultrastructural resolution (2nm/px) using transmission electron microscopy. Postsynaptic partners of CBb6 cell 6156’s presynaptic structures were annotated using the Viking Viewer for Connectomics. Statistical analyses were conducted in Microsoft Excel and investigated further with 3D rendering and graph visualization of connectivity.
Results
The factors tracked for comparison included presynaptic structure type, target number, and postsynaptic partner type. Multiribbon synapses of CBb6 cell 6156 trended towards having a greater number of output partners, with a greater proportion of dyads than monads. Despite this, triads and quadrads were only found opposing single ribbon synapses. As the different presynaptic structure types may differ in the strength of neurotransmitter release (ribbonless < single ribbon < multiribbon), these findings are inconsistent with scaling of output to the number of postsynaptic targets. Both amacrine cells (AC) and ganglion cells (GC) are postsynaptic partners of 6156. However, single ribbon and ribbonless structures appear biased towards AC only targets, while multiribbon synapses appear biased toward mixed AC and GC targets.
Conclusions
Target type relationships appear more important than the number of targets in determining presynaptic structure type in CBb6. Future efforts will examine size differences of postsynaptic structures and presynaptic ribbon size, and even compare across bipolar cell classes, in order to provide further insight on the connectivity rules underlying excitatory synapses.