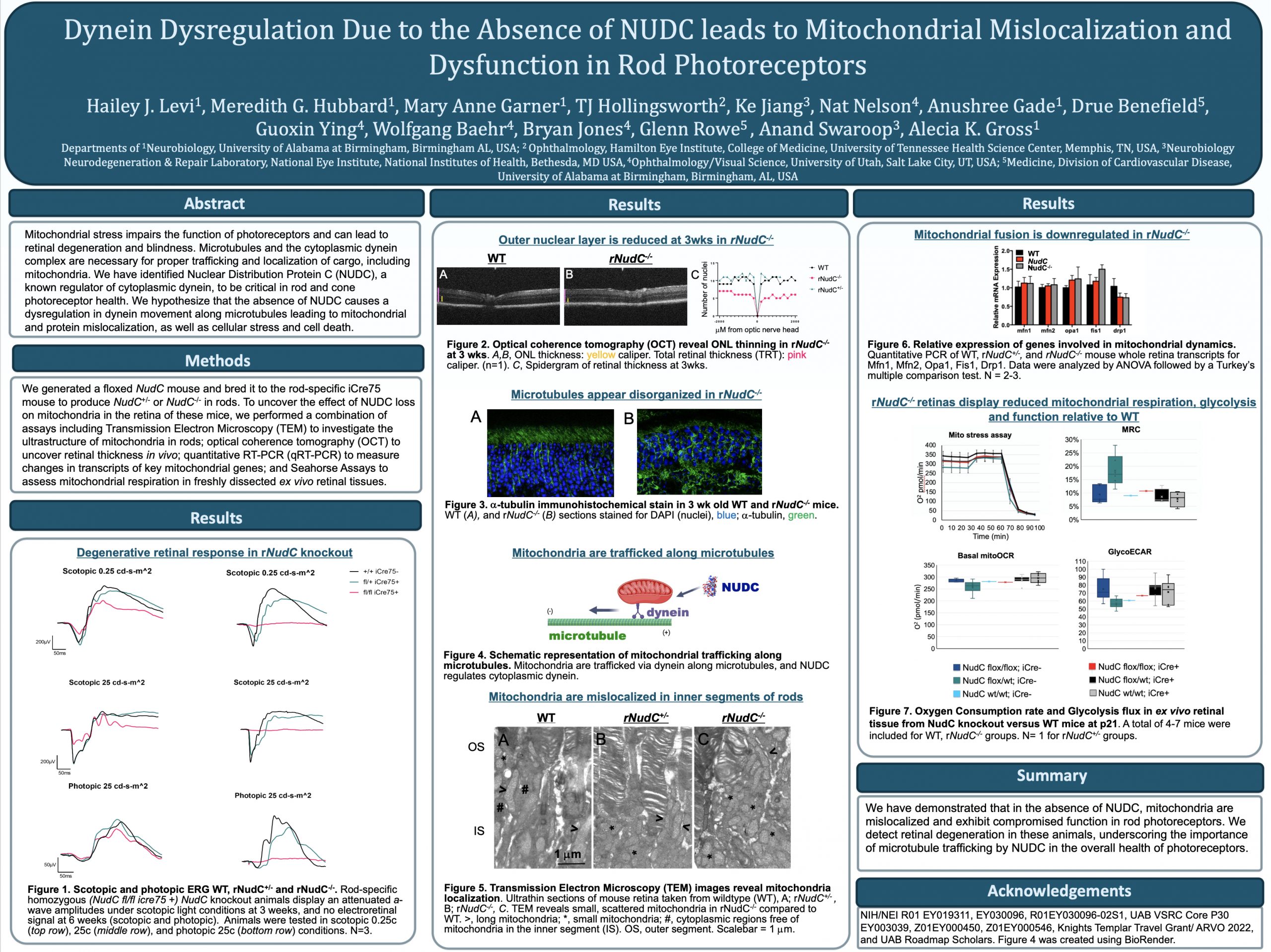
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Hailey Levi @drpepperis100, Meredith Hubbard, Mary Anne Garner, TJ Hollingsworth, Ke Jiang, Nat Nelson, Anushree Gade, Drue Benefield, Guoxin Ying, Wolfgang Baehr, Bryan Jones@BWJones, Anand Swaroop, Glenn Rowe, and Alecia Gross @alecia144g.
Tag Archives: ARVO
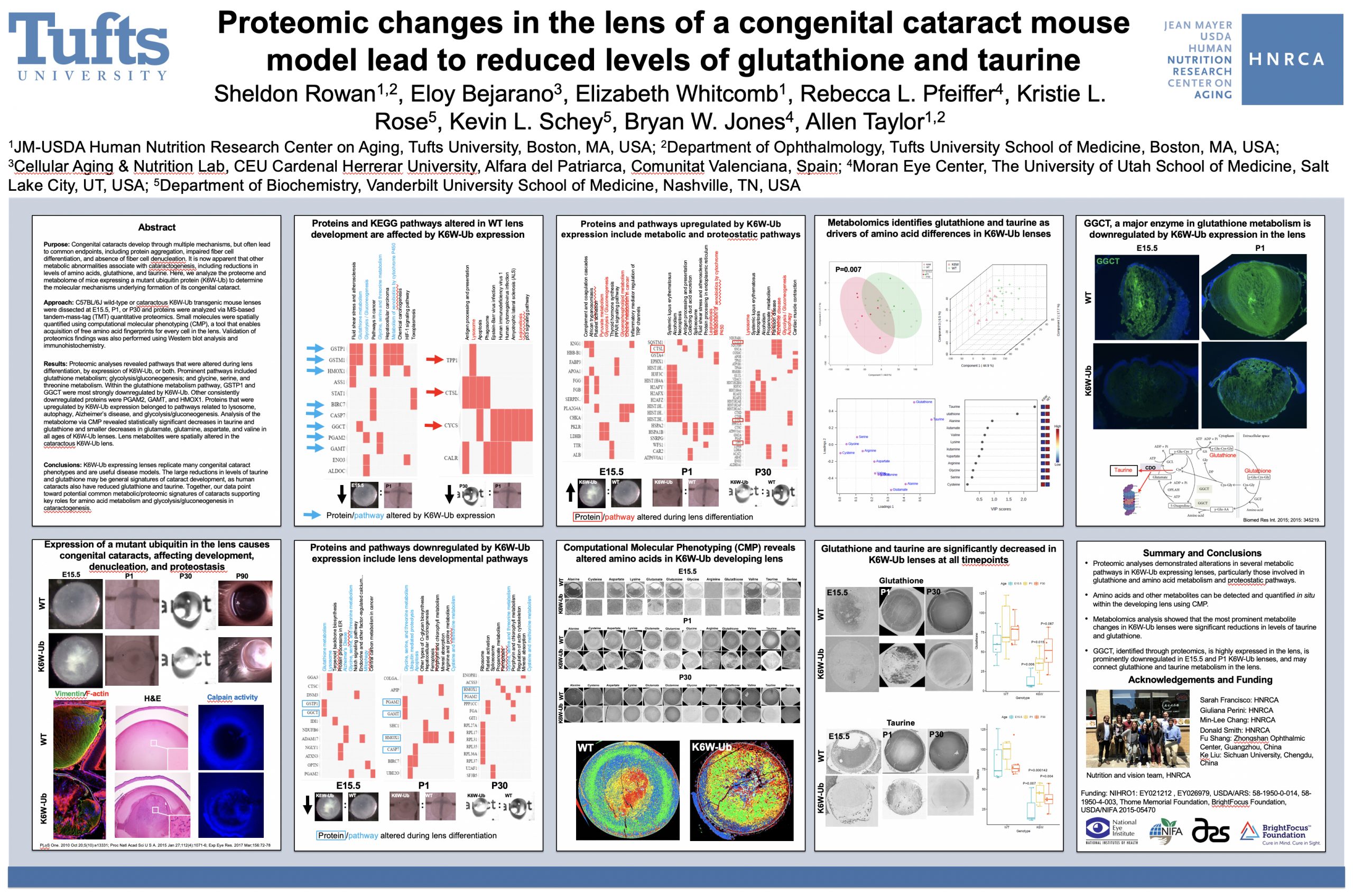
Proteomic changes in the lens of a congenital cataract mouse model lead to reduced levels of glutathione and taurine
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Sheldon Rowan @SheldonRowan, Eloy Bejarano, Elizabeth Whitcomb, Rebecca Pfeiffer @BeccaPfeiffer19, Kristie Rose, Kevin Schey, Bryan Jones @BWJones, Allen Taylor.
Purpose: Congenital cataracts develop through multiple mechanisms, but often lead to common endpoints, including protein aggregation, impaired fiber cell differentiation, and absence of fiber cell denucleation. It is now apparent that other metabolic abnormalities associate with cataractogenesis, including reductions in levels of amino acids, glutathione, and taurine. Here, we analyze the proteome and metabolome of mice expressing a mutant ubiquitin protein (K6W-Ub) to determine the molecular mechanisms underlying formation of its congenital cataract.
Methods: C57BL/6J wild-type or cataractous K6W-Ub transgenic mouse lenses were dissected at E15.5, P1, or P30 and proteins were analyzed via MS-based tandem-mass-tag (TMT) quantitative proteomics. Small molecules were spatially quantified using computational molecular phenotyping (CMP), a tool that enables acquisition of free amino acid fingerprints for every cell in the lens. Validation of proteomics findings was also performed using Western blot analysis and immunohistochemistry.
Results: Proteomic analyses revealed pathways that were altered during lens differentiation, by expression of K6W-Ub, or both. Prominent pathways included glutathione metabolism; glycolysis/gluconeogenesis; and glycine, serine, and threonine metabolism. Within the glutathione metabolism pathway, GSTP1 and GGCT were most strongly downregulated by K6W-Ub. Other consistently downregulated proteins were PGAM2, GAMT, and HMOX1. Proteins that were upregulated by K6W-Ub expression belonged to pathways related to lysosome, autophagy, Alzheimer’s disease, and glycolysis/gluconeogenesis. Analysis of the metabolome via CMP revealed statistically significant decreases in taurine and glutathione and smaller decreases in glutamate, glutamine, aspartate, and valine in all ages of K6W-Ub lenses. Lens metabolites were spatially altered in the cataractous K6W-Ub lens.
Conclusions: K6W-Ub expressing lenses replicate many congenital cataract phenotypes and are useful disease models. The large reductions in levels of taurine and glutathione may be general signatures of cataract development, as human cataracts also have reduced glutathione and taurine. Key roles for amino acid metabolism and glycolysis/gluconeogenesis in cataractogenesis are emerging. Together our data point toward potential common metabolic/proteomic signatures of cataracts.
ARVO Mini-Symposium: Pathoconnectomics in Retinal Degeneration
Lab PI, Bryan Jones delivered a talk at the ARVO 2022 mini-symposium on Pathoconnectomics in Retinal Degeneration.
Abstract: Connectomics has demonstrated that synaptic networks and their topologies are precise and directly correlate with physiology and behavior. The next extension of connectomics is pathoconnectomics: to map neural network synaptology and circuit topologies corrupted by neurological disease in order to identify robust targets for therapeutics. The retina is ideal for pathoconnectomics approaches, and reveals common rules of how neural systems are wired, and how they break in neurodegenerative disease.
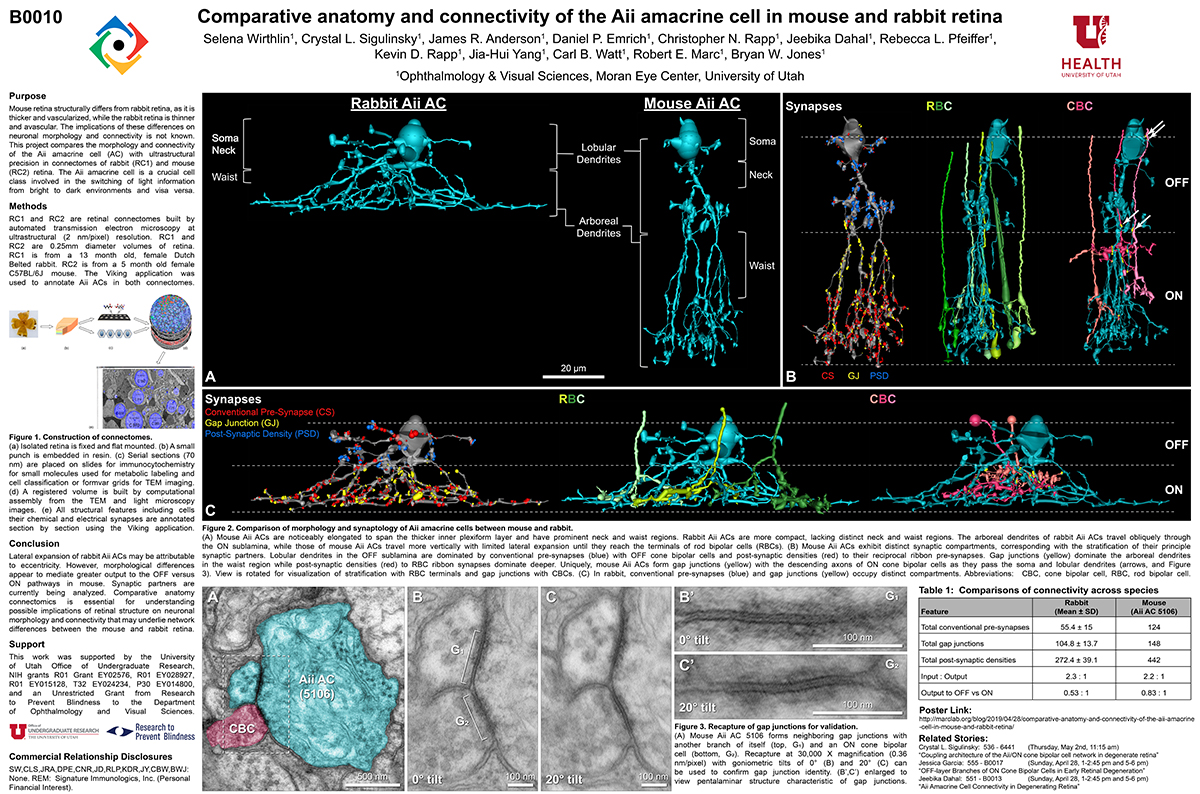
Comparative Anatomy And Connectivity Of The AII Amacrine Cell In Mouse And Rabbit Retina
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Selena Wirthlin, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Christopher Rapp, Jeebika Dahal, Rebecca L. Pfeiffer, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose
Mouse retina structurally differs from rabbit retina, as it is thicker and vascularized, while the rabbit retina is thinner and avascular. The implications of these differences on neuronal morphology and connectivity is not known. This project compares the morphology and connectivity of the Aii amacrine cell (AC) with ultrastructural precision in connectomes of mouse (RC2) and rabbit (RC1) retina.
Methods
RC1 and RC2 are connectomes built by automated transmission electron microscopy at ultrastructural (2 nm/pixel) resolution. RC1 and RC2 are 0.25mm diameter volumes of retina. RC1 is from a 13 month old, female Dutch Belted rabbit. RC2 is from a 5 month old female C57BL/6J mouse. The Viking application was used to annotate Aii ACs in both connectomes.
Results
Mouse Aii ACs are noticeably elongated to span the thicker inner plexiform layer (IPL) and have a prominent neck region. Lobular appendages of Aii ACs in both species extend thin stalks from the soma, neck and proximal arboreal dendrites in the OFF sublamina, predominantly forming reciprocal synapses with OFF cone bipolar cells (BCs). In rabbits, multiple arboreal dendrites emerge from the base of the neck, branch and travel obliquely through the ON sublamina, and form gap junctions with ON cone BCs, neighbor Aii ACs, and itself. They extend laterally at the base of the IPL, collecting ribbon input from rod BCs. In contrast, mouse arboreal dendrites stem from a single primary dendrite that branches as it travels vertically through the IPL without self-branch interaction, terminating at variable depths that align with the more broadly ramified axon terminals of rod BCs. Conventional synapse to gap junction ratios reveal greater output in the OFF vs ON layer in mouse compared to rabbit. Notably, mouse Aii ACs form gap junctions with the descending axons of ON cone BCs as they pass its soma, in contrast to rabbit, where gap junctions do not form at contacts proximal to ON cone BC axon terminals.
Conclusions
Lateral expansion of rabbit Aii ACs may be attributable to eccentricity. However, morphological differences appear to mediate greater output to the OFF versus ON pathway in mouse. Synaptic partners are currently being analyzed. Comparative anatomy connectomics is essential for understanding possible implications of retinal structure on neuronal morphology and connectivity that may underlie network differences between the mouse and rabbit retina.
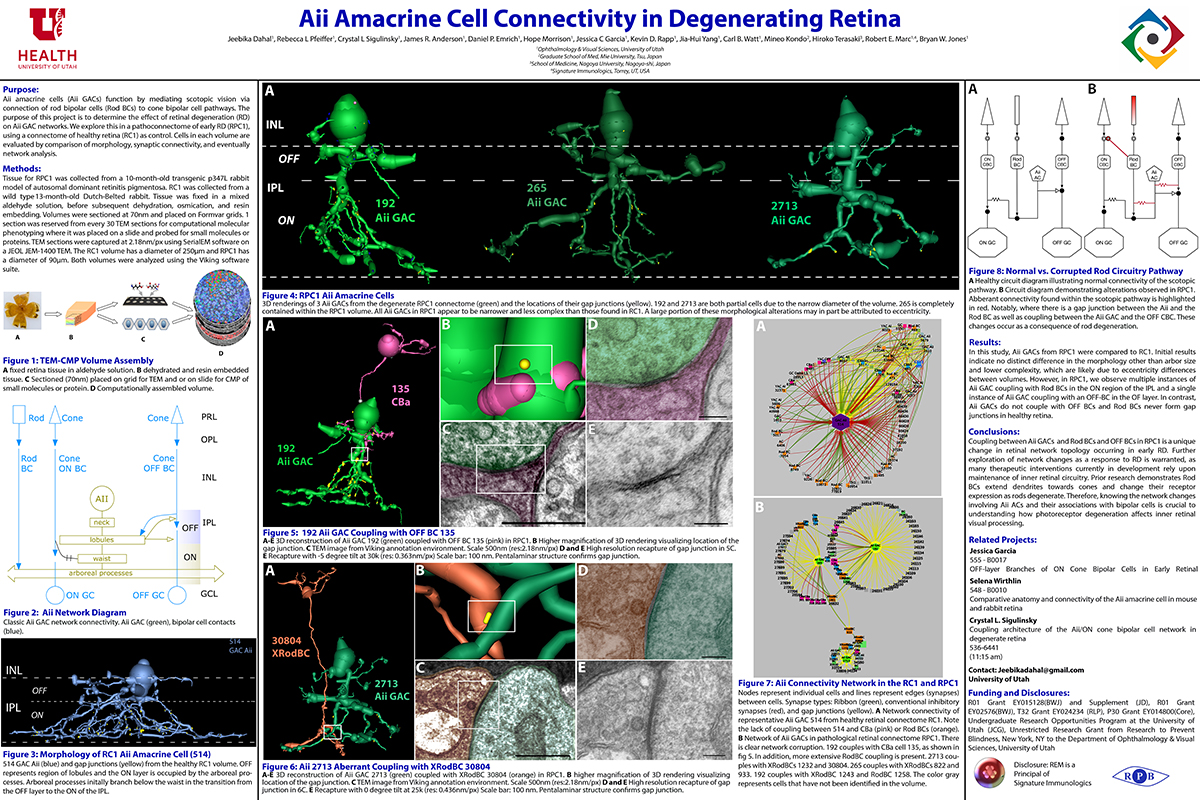
Aii Amacrine Cell Connectivity in Degenerating Retina
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Jeebika Dahal, Rebecca L. Pfeiffer, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Hope Morrison, Jessica C. Garcia, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Mineo Kondo, Hiroko Terasaki, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose
Aii amacrine cells (Aii ACs) function in mediating scotopic vision via connection of rod bipolar cells (Rod BCs) to cone bipolar cell pathways. The purpose of this project is to determine the effect of retinal degeneration (RD) on Aii AC networks. We explore this in a pathoconnectome of early RD (RPC1), using a connectome of healthy retina (RC1) as control. Cells in each volume are evaluated by comparison of morphology, synaptic connectivity, and eventually network analysis.
Methods
Tissue for RPC1 was collected from a 10 month old transgenic p347L rabbit model of autosomal dominant retinitis pigmentosa. RC1 was collected from a 13 month old Dutch-Belted rabbit, with no indications of degeneration. Tissue was fixed in a mixed aldehyde solution, before subsequent dehydration, osmication, and resin embedding. Volumes were sectioned at 70nm (RPC1) and 90nm (RC1) and placed on formvar grids. 1 section was reserved from every 30 TEM sections for computational molecular phenotyping where it was placed on a slide and probed for small molecules or proteins. TEM sections were captured at 2.18nm/px using SerialEM software on a JEOL JEM-1400 TEM. The RC1 volume has a diameter of 250µm and RPC1 has a diameter of 90µm. Both volumes were analyzed using the Viking software suite.
Results
In this study, Aii ACs from RPC1 were compared to RC1. Initial results indicate no distinct difference in the morphology other than arbor size, which are likely due to eccentricity differences between volumes. However, in RPC1, we observe multiple instances of Aii AC coupling with Rod BCs in the ON region of the IPL. In contrast, Rod BCs never form gap junctions in healthy retina.
Conclusions
Coupling between Aii ACs and Rod BCs in RPC1 is a unique change in retinal network topology occurring in early RD. Further exploration of network changes as a response to RD is warranted, as many therapeutic interventions currently in development rely upon maintenance of inner retinal circuitry. Prior research demonstrates Rod BCs extend dendrites towards cones and change their receptor expression as rods degenerate. Therefore, knowing the network changes involving Aii ACs and their associations with bipolar cells is crucial to understanding how photoreceptor degeneration affects inner retinal visual processing.
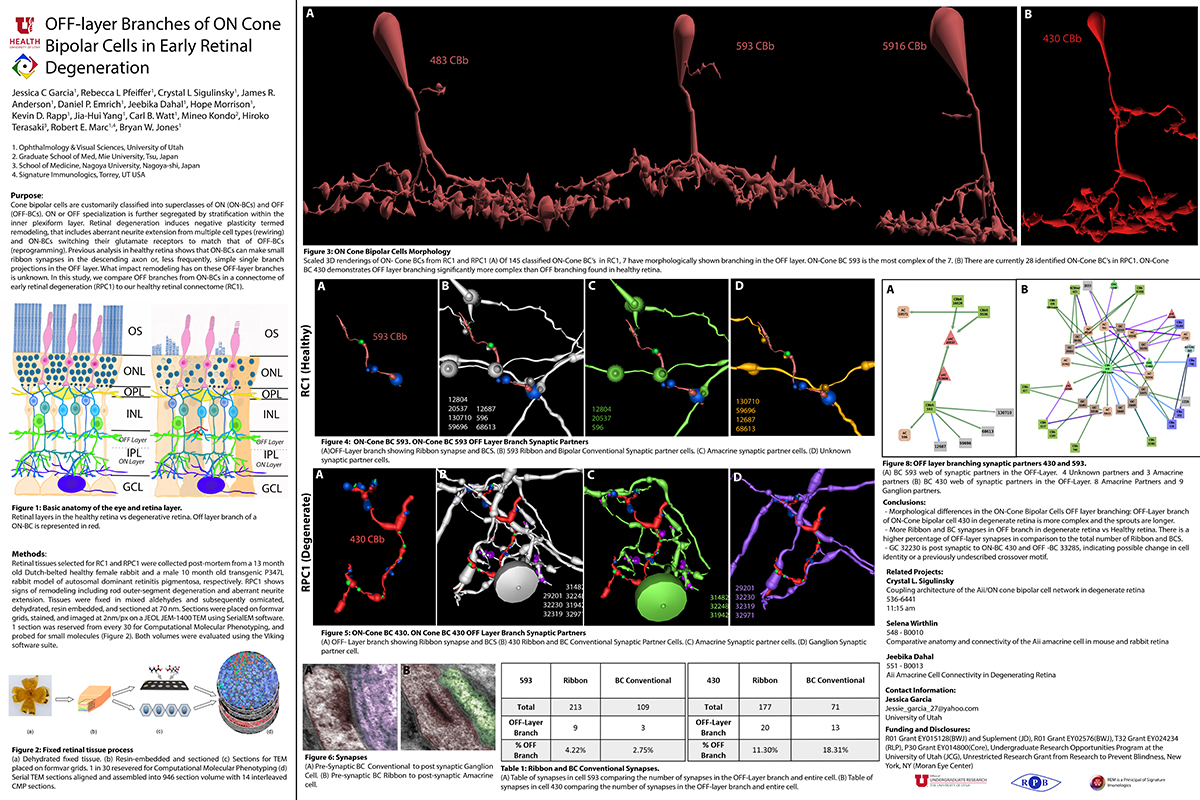
OFF-layer Branches of ON Cone Bipolar Cells in Early Retinal Degeneration
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Jessica C. Garcia, Rebecca L. Pfeiffer, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Jeebika Dahal, Hope Morrison, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Mineo Kondo, Hiroko Terasaki, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose: Cone bipolar cells are customarily classified into superclasses of ON (ON-BCs) and OFF (OFF-BCs). ON or OFF specialization is further segregated by stratification within the inner plexiform layer. Retinal degeneration induces negative plasticity termed remodeling, that includes aberrant neurite extension from multiple cell types (rewiring) and ON-BCs switching their glutamate receptors to match that of OFF-BCs (reprogramming). Previous analysis in healthy retina shows that ON-BCs can make small ribbon synapses in the descending axon or, less frequently, simple single branch projections in the OFF layer. What impact remodeling has on these OFF-layer branches is unknown. In this study, we compare OFF branches from ON-BCs in a connectome of early retinal degeneration (RPC1) to our healthy retinal connectome (RC1).
Methods: Retinal tissues selected for RC1 and RPC1 were collected post-mortem from a 13 month old Dutch-belted healthy female rabbit and a male 10 month old transgenic P347L rabbit model of autosomal dominant retinitis pigmentosa, respectively. RPC1 shows signs of remodeling including rod outer-segment degeneration and aberrant neurite extension. Tissues were fixed in mixed aldehydes and subsequently osmicated, dehydrated, resin embedded, and sectioned at 90 nm (RC1) or 70 nm (RPC1). Sections were placed on formvar grids, stained, and imaged at 2nm/px on a JEOL JEM-1400 TEM using SerialEM software. 1 section was reserved from every 30 for Computational Molecular Phenotyping, and probed for small molecules. Both volumes were evaluated using the Viking software suite.
Results: Ribbons of ON-BCs formed in the OFF layer branches have been previously described to contact glycinergic amacrine cells (ACs), GABAergic ACs, ON ganglion cells, and intrinsically photosensitive ganglion cells. Initial analysis of OFF branches of ON-BCs in RPC1 demonstrate more complex branching than in RC1 and increased number of synapses on these branches. In contrast to the inconsistent OFF layer branch stratification observed in RC1, the OFF branches in RPC1 appear to stratify at a similar level. Evaluation of synaptic partners is ongoing.
Conclusions: Increased complexity and number of synapses found in the OFF branches of some ON-BCs ultimately may represent ON network corruption. Exploring synaptic partners will reveal potential network alterations in retinal degenerative disease.
ARVO 2019 Prep
The annual ARVO meeting is almost here and we are in full preparation mode for the meeting. This image shows postdoc Rebecca Pfeiffer (@BeccaPfeiffer19) with undergraduate (and US Navy veteran) Jessica Garcia preparing data for presentation.
Circuit Remodeling In Retinal Degeneration
This abstract was presented yesterday, April 29th at the 2018 Association for Research in Vision and Opthalmology (ARVO) meetings in Honolulu, Hawaii by Bryan W. Jones.
Abstract:
The retina is a complex, heterocellular tissue with most/all retinal cell classes becoming impacted or altered in retinitis pigmentosa (RP) and age-related macular degeneration (AMD) in a process called retinal remodeling. Defining disease and the stage-specific cytoarchitectural and metabolic responses in RP and AMD is critical for highlighting targets for intervention. We now know that negative plasticity and neural retinal remodeling occurs regardless of retinal insult in models of retinal degeneration as well as in human RP and in human AMD, revealing that no retinal disease fails to trigger remodeling and reprogramming.
Evidence in the literature over the past decade has improved our understanding into mechanisms of initial retinal degeneration and informed our understanding of the subsequent remodeling events in the neural retina that occur post-photoreceptor degeneration. Remodeling associated with retinal degeneration is intimately linked with insults that cause photoreceptor stress and eventually photoreceptor cell death. These phenomena result in reprogramming of cell types in retina followed by progressive neural degeneration akin to CNS neural degenerations involving both neuronal and glial classes. No cell class in the retina is spared from the effects of remodeling. The earliest cell classes involved in remodeling are horizontal, bipolar and Müller cells and the Müller glia are the last cell class left in the remodeling retina.
Our efforts are now focused on elucidating the precise wiring changes in retina, through the creation of pathological connectomes, or “patho-connectomes” to study precisely what the circuit topologies are, compared to normal topologies derived from Retinal Connectome 1 (RC1). Also, because temporal windows are critical to understanding when interventions may be possible, we are exploring when circuit topology revisions occur to understand their impact on information flow in the retina and their impact on rescues of vision loss. Precise circuit topologies in early retinal degenerative events is our first area of exploration with ultrastructural reconstructions of outer retinal neurons, bipolar cells and horizontal cells. Müller glia are also of intense interest as we are tracking the earliest metabolic and morphological changes in glia in response to retinal degenerations.
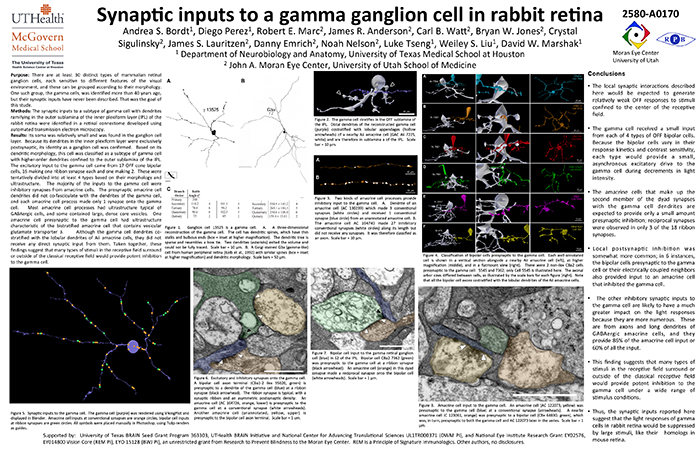
Synaptic Inputs To A Gamma Ganglion Cell In Rabbit Retina
This abstract was presented today, May 8th at the 2017 Association for Research in Vision and Opthalmology (ARVO) meetings in Baltimore, Maryland by Andrea Bordt, Diego Perez, Robert E. Marc, James R. Anderson, Carl B. Watt, Bryan W. Jones, Crystal Sigulinsky, James S. Lauritzen, Danny Emrich, Noah Nelson, Luke Tseng, Weiley Liu, and David W. Marshak. Full resolution version here.
Purpose: There are at least 30 distinct types of mammalian retinal ganglion cells, each sensitive to different features of the visual environment, and these can be grouped according to their morphology. One such group, the gamma cells, was identified more than 40 years ago, but their synaptic inputs have never been described. That was the goal of this study.
Methods: The synaptic inputs to a subtype of gamma cell with dendrites ramifying in the outer sublamina of the inner plexiform layer (IPL) of the rabbit retina were identified in a retinal connectome developed using automated transmission electron microscopy.
Results: The gamma cell was always postsynaptic in the IPL, confirming its identity as a ganglion cell. The local synaptic input should produce relatively weak OFF reposnses to stimuli confined to the center of the gamma cell’s receptive field. It typically received only one synapse per bipolar cell from at least 4 types of OFF bipolar cells. Because bipolar cells vary in their response kinetics and contrast sensitivity. each type would provide a small, asynchronous excitatory input. The amacrine cells at the dyad synapses provided only a small amount presynaptic inhibition; reciprocal synapses were observed in only 3 of the 18 ribbon synapses. There was no glycinergic crossover inhibition, another local interaction that would enhance light responses. Local postsynaptic inhibition was somewhat more common; in 6 instances, the bipolar cells presynaptic to the gamma cell or their electrically coupled neighbors also provided input to an amacrine cell that inhibited the gamma cell. The other amacrine cell inputs to the gamma cell should have a much greater impact on the light responses because they are far more numerous. These are from axons and long dendrites of GABAergic amacrine cells, and they provide 60% of all the input. This finding suggests that many types of stimuli in the receptive field surround or outside of the classical receptive field would provide potent inhibition to the gamma cell.
Conclusions: The synaptic inputs rsuggest that gamma cells in rabbit retina would have light responses like their homologs in mouse retina, OFF responses to small stimuli in the receptive field center that are suppressed by a variety of larger stimuli. Thus, they would signal object motion selectively.
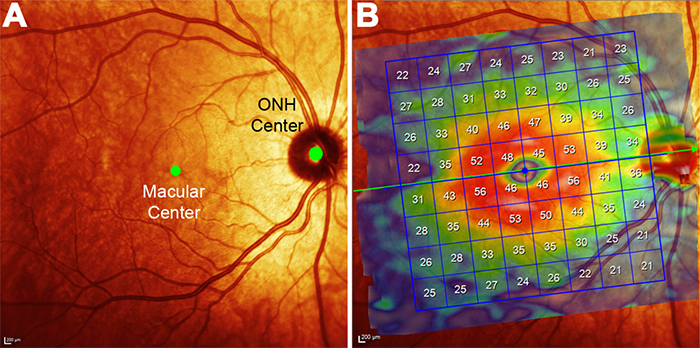
Predicting Age-related Changes with High Accuracy using a Pattern Recognition Derived Retinal Ganglion Cell Regression Model
This abstract was presented yesterday, May 7th at the 2017 Association for Research in Vision and Opthalmology (ARVO) meetings in Baltimore, Maryland by Nayuta Yoshioka, Barbara Zangerl, Lisa Nivison-Smith, Sieu Khuu, Bryan W. Jones, Rebecca Pfeiffer, Robert Marc, and Michael Kalloniatis.
Purpose: We recently used pattern recognition analysis to show macula areas can be classified into statistically distinct clusters in accordance to their age-related retinal ganglion cell layer (RGCL) thickness change in a normal population. The aim of this study was to perform a retrospective cross-sectional analysis utilizing a large cohort of patients to establish accuracy of this model and to develop a normative dataset using a 50-year-old equivalent cohort.
Methods: Data was collected from patients seen at the Centre for Eye Health for optic nerve assessment without posterior pole disease. The grid-wise RGCL thickness was obtained from a single eye of each patient via Spectralis OCT macular scan over an 8×8 measurement grid. Measurements for patients outside the 45-54 age range (training cohort) were converted to 50-year-old equivalent value utilizing pattern recognition derived regression model which, in brief, consists of 8×8 grid clustered into 8 distinct classes according to the pattern of RGCL thickness change with age. Accuracy of the predictions was assessed by comparing the training cohort’s measurements to the 45-54 year reference cohort using t-test and one-way ANOVA.
Results: Data were collected from a total 248 patients aged 20 to 78.1 years. 80 patients within this group were aged 45 – 54 and formed the reference cohort (average±SD 49.6±2.83) and the remaining 168 eyes formed the training cohort (average age±SD 50.7±17.34). Converted values for the training set matched those of the reference cohort (average disparity±SD 0.10±0.42µm, range -0.74-1.34µm) and were not significantly different (p > 0.9). Most variability was observed with patients above 70 years of age (average disparity±SD -0.09±1.73µm, range -3.67 to 6.16µm) and central grids corresponding to the fovea (average disparity±SD 0.47±0.72µm, range -0.22 to 1.34µm).
Conclusions: Our regression model for normal age-related RGCL change can accurately convert and/or predict RGCL thickness for individuals in comparison to 50-year-equivalent reference cohort and could allow for more accurate assessment of RGCL thickness and earlier detection of significant loss in the future. Caution may be needed when applying the model in the foveal area or for patients older than 70 years.