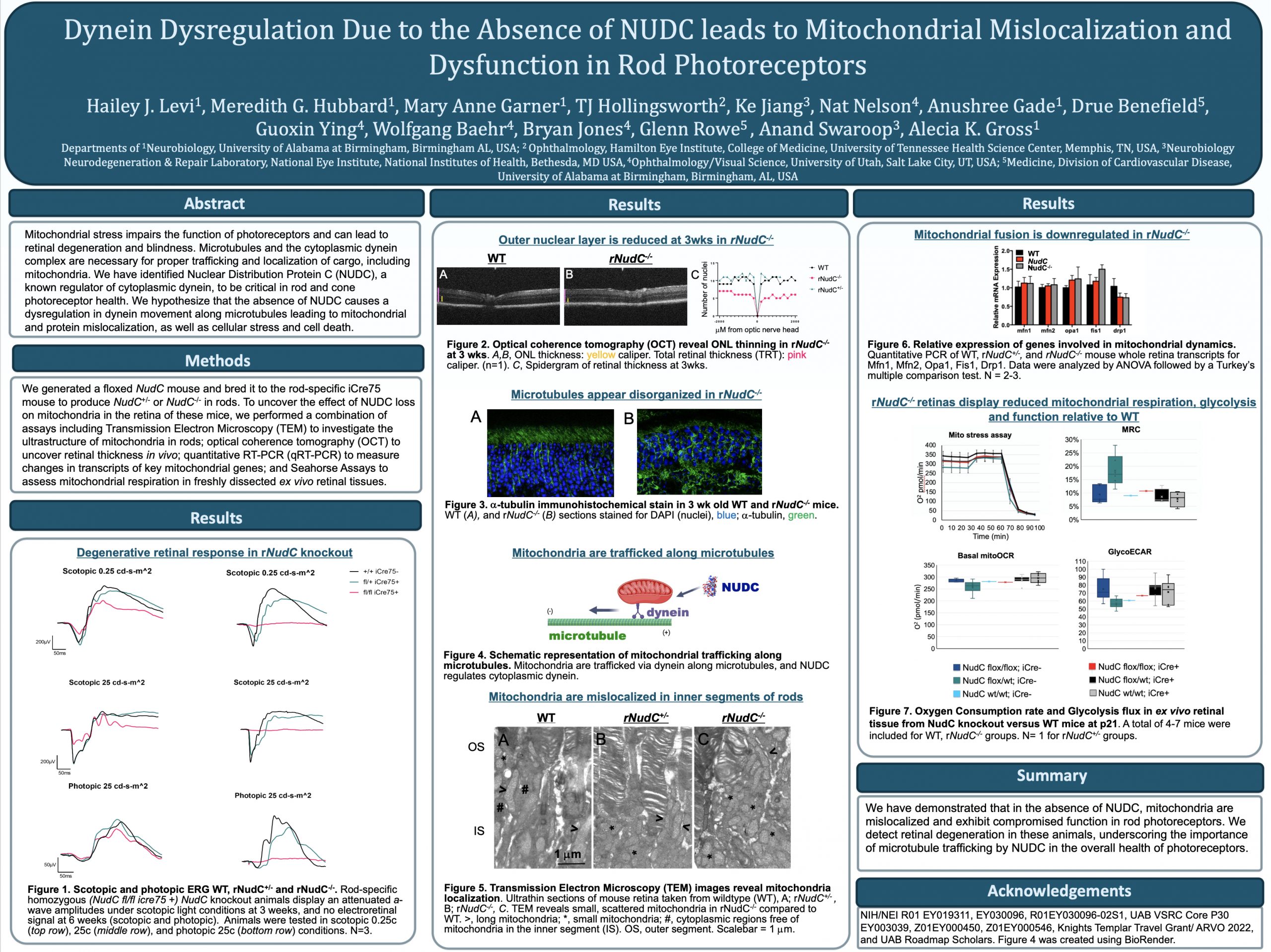
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Hailey Levi @drpepperis100, Meredith Hubbard, Mary Anne Garner, TJ Hollingsworth, Ke Jiang, Nat Nelson, Anushree Gade, Drue Benefield, Guoxin Ying, Wolfgang Baehr, Bryan Jones@BWJones, Anand Swaroop, Glenn Rowe, and Alecia Gross @alecia144g.
Tag Archives: poster
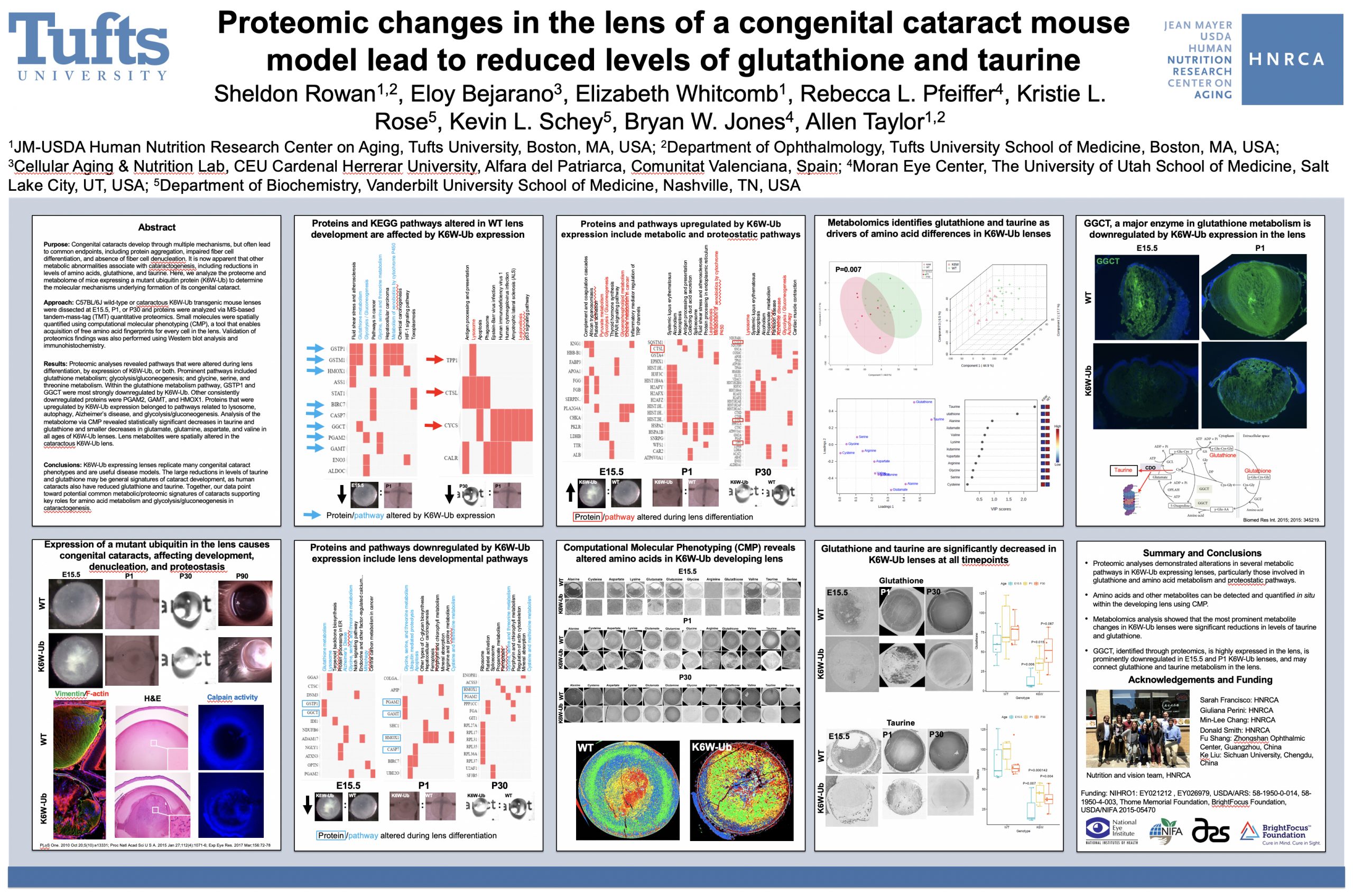
Proteomic changes in the lens of a congenital cataract mouse model lead to reduced levels of glutathione and taurine
This abstract was presented today, May 4th at the 2022 Association for Research in Vision and Opthalmology (ARVO) meetings in Denver, Colorado by Sheldon Rowan @SheldonRowan, Eloy Bejarano, Elizabeth Whitcomb, Rebecca Pfeiffer @BeccaPfeiffer19, Kristie Rose, Kevin Schey, Bryan Jones @BWJones, Allen Taylor.
Purpose: Congenital cataracts develop through multiple mechanisms, but often lead to common endpoints, including protein aggregation, impaired fiber cell differentiation, and absence of fiber cell denucleation. It is now apparent that other metabolic abnormalities associate with cataractogenesis, including reductions in levels of amino acids, glutathione, and taurine. Here, we analyze the proteome and metabolome of mice expressing a mutant ubiquitin protein (K6W-Ub) to determine the molecular mechanisms underlying formation of its congenital cataract.
Methods: C57BL/6J wild-type or cataractous K6W-Ub transgenic mouse lenses were dissected at E15.5, P1, or P30 and proteins were analyzed via MS-based tandem-mass-tag (TMT) quantitative proteomics. Small molecules were spatially quantified using computational molecular phenotyping (CMP), a tool that enables acquisition of free amino acid fingerprints for every cell in the lens. Validation of proteomics findings was also performed using Western blot analysis and immunohistochemistry.
Results: Proteomic analyses revealed pathways that were altered during lens differentiation, by expression of K6W-Ub, or both. Prominent pathways included glutathione metabolism; glycolysis/gluconeogenesis; and glycine, serine, and threonine metabolism. Within the glutathione metabolism pathway, GSTP1 and GGCT were most strongly downregulated by K6W-Ub. Other consistently downregulated proteins were PGAM2, GAMT, and HMOX1. Proteins that were upregulated by K6W-Ub expression belonged to pathways related to lysosome, autophagy, Alzheimer’s disease, and glycolysis/gluconeogenesis. Analysis of the metabolome via CMP revealed statistically significant decreases in taurine and glutathione and smaller decreases in glutamate, glutamine, aspartate, and valine in all ages of K6W-Ub lenses. Lens metabolites were spatially altered in the cataractous K6W-Ub lens.
Conclusions: K6W-Ub expressing lenses replicate many congenital cataract phenotypes and are useful disease models. The large reductions in levels of taurine and glutathione may be general signatures of cataract development, as human cataracts also have reduced glutathione and taurine. Key roles for amino acid metabolism and glycolysis/gluconeogenesis in cataractogenesis are emerging. Together our data point toward potential common metabolic/proteomic signatures of cataracts.
ARVO Mini-Symposium: Pathoconnectomics in Retinal Degeneration
Lab PI, Bryan Jones delivered a talk at the ARVO 2022 mini-symposium on Pathoconnectomics in Retinal Degeneration.
Abstract: Connectomics has demonstrated that synaptic networks and their topologies are precise and directly correlate with physiology and behavior. The next extension of connectomics is pathoconnectomics: to map neural network synaptology and circuit topologies corrupted by neurological disease in order to identify robust targets for therapeutics. The retina is ideal for pathoconnectomics approaches, and reveals common rules of how neural systems are wired, and how they break in neurodegenerative disease.
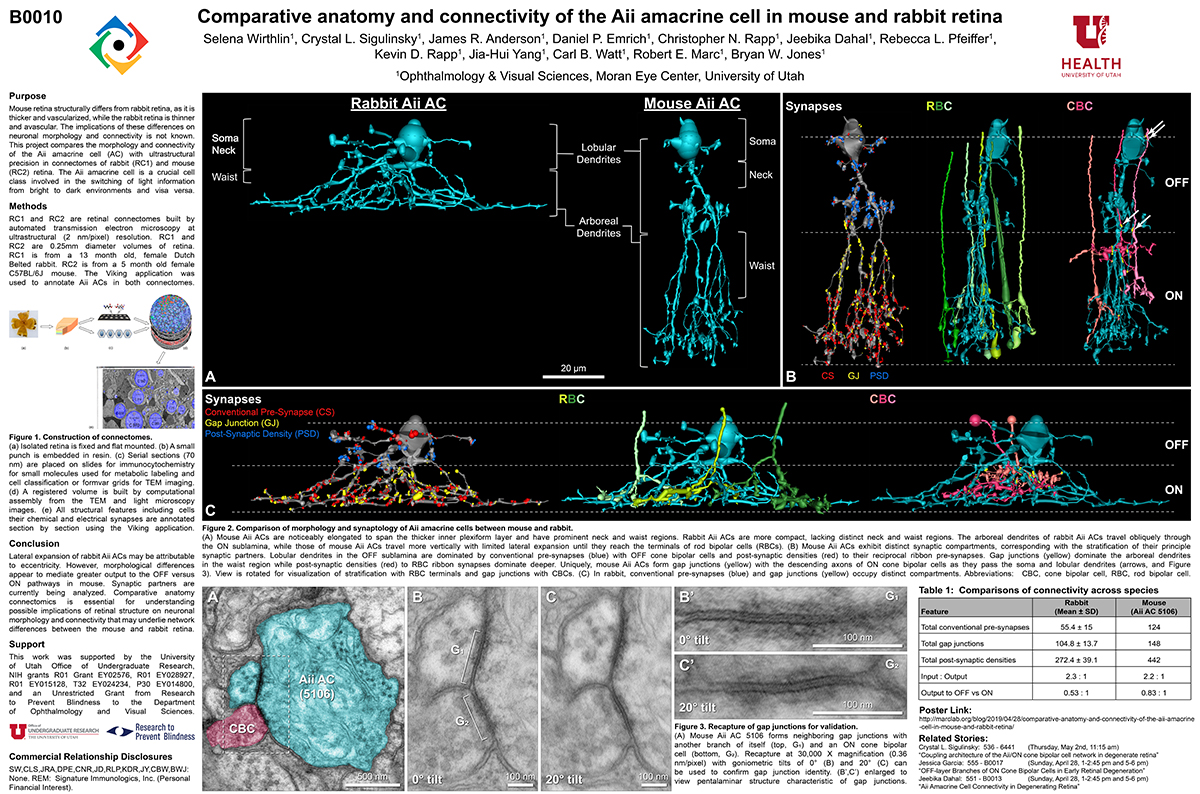
Comparative Anatomy And Connectivity Of The AII Amacrine Cell In Mouse And Rabbit Retina
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Selena Wirthlin, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Christopher Rapp, Jeebika Dahal, Rebecca L. Pfeiffer, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose
Mouse retina structurally differs from rabbit retina, as it is thicker and vascularized, while the rabbit retina is thinner and avascular. The implications of these differences on neuronal morphology and connectivity is not known. This project compares the morphology and connectivity of the Aii amacrine cell (AC) with ultrastructural precision in connectomes of mouse (RC2) and rabbit (RC1) retina.
Methods
RC1 and RC2 are connectomes built by automated transmission electron microscopy at ultrastructural (2 nm/pixel) resolution. RC1 and RC2 are 0.25mm diameter volumes of retina. RC1 is from a 13 month old, female Dutch Belted rabbit. RC2 is from a 5 month old female C57BL/6J mouse. The Viking application was used to annotate Aii ACs in both connectomes.
Results
Mouse Aii ACs are noticeably elongated to span the thicker inner plexiform layer (IPL) and have a prominent neck region. Lobular appendages of Aii ACs in both species extend thin stalks from the soma, neck and proximal arboreal dendrites in the OFF sublamina, predominantly forming reciprocal synapses with OFF cone bipolar cells (BCs). In rabbits, multiple arboreal dendrites emerge from the base of the neck, branch and travel obliquely through the ON sublamina, and form gap junctions with ON cone BCs, neighbor Aii ACs, and itself. They extend laterally at the base of the IPL, collecting ribbon input from rod BCs. In contrast, mouse arboreal dendrites stem from a single primary dendrite that branches as it travels vertically through the IPL without self-branch interaction, terminating at variable depths that align with the more broadly ramified axon terminals of rod BCs. Conventional synapse to gap junction ratios reveal greater output in the OFF vs ON layer in mouse compared to rabbit. Notably, mouse Aii ACs form gap junctions with the descending axons of ON cone BCs as they pass its soma, in contrast to rabbit, where gap junctions do not form at contacts proximal to ON cone BC axon terminals.
Conclusions
Lateral expansion of rabbit Aii ACs may be attributable to eccentricity. However, morphological differences appear to mediate greater output to the OFF versus ON pathway in mouse. Synaptic partners are currently being analyzed. Comparative anatomy connectomics is essential for understanding possible implications of retinal structure on neuronal morphology and connectivity that may underlie network differences between the mouse and rabbit retina.
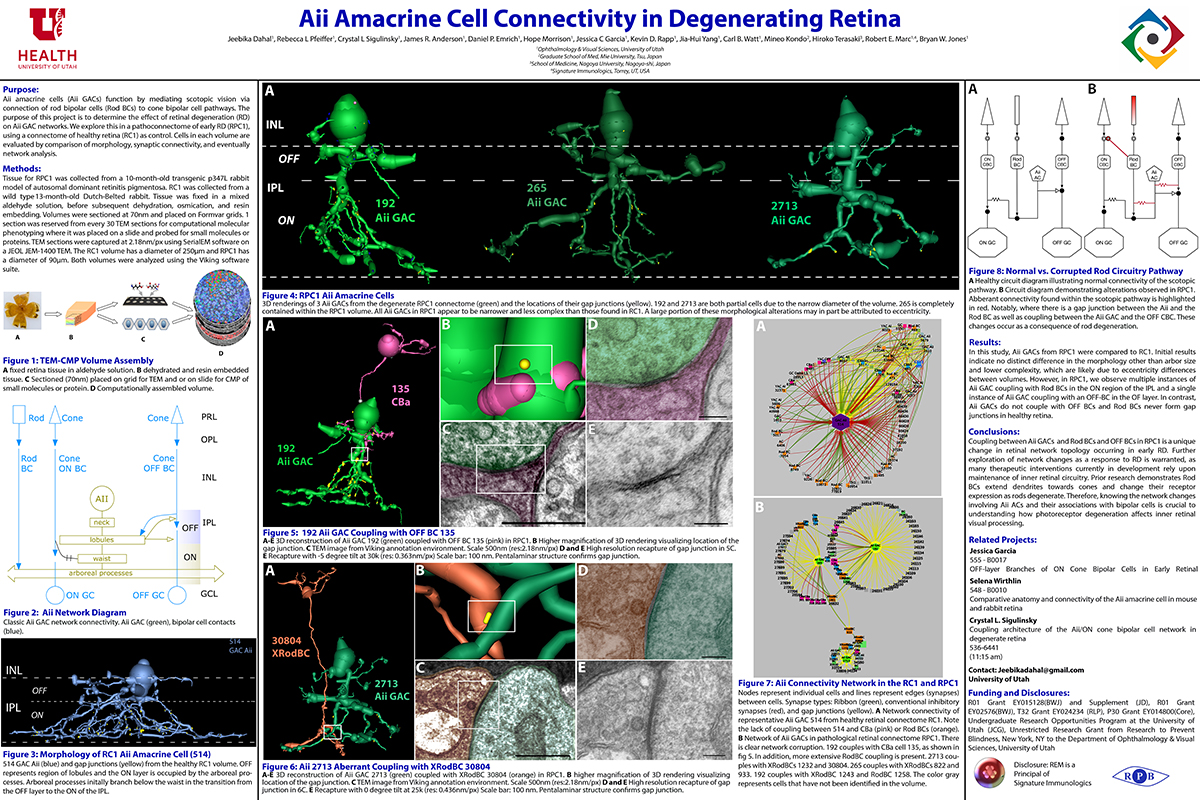
Aii Amacrine Cell Connectivity in Degenerating Retina
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Jeebika Dahal, Rebecca L. Pfeiffer, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Hope Morrison, Jessica C. Garcia, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Mineo Kondo, Hiroko Terasaki, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose
Aii amacrine cells (Aii ACs) function in mediating scotopic vision via connection of rod bipolar cells (Rod BCs) to cone bipolar cell pathways. The purpose of this project is to determine the effect of retinal degeneration (RD) on Aii AC networks. We explore this in a pathoconnectome of early RD (RPC1), using a connectome of healthy retina (RC1) as control. Cells in each volume are evaluated by comparison of morphology, synaptic connectivity, and eventually network analysis.
Methods
Tissue for RPC1 was collected from a 10 month old transgenic p347L rabbit model of autosomal dominant retinitis pigmentosa. RC1 was collected from a 13 month old Dutch-Belted rabbit, with no indications of degeneration. Tissue was fixed in a mixed aldehyde solution, before subsequent dehydration, osmication, and resin embedding. Volumes were sectioned at 70nm (RPC1) and 90nm (RC1) and placed on formvar grids. 1 section was reserved from every 30 TEM sections for computational molecular phenotyping where it was placed on a slide and probed for small molecules or proteins. TEM sections were captured at 2.18nm/px using SerialEM software on a JEOL JEM-1400 TEM. The RC1 volume has a diameter of 250µm and RPC1 has a diameter of 90µm. Both volumes were analyzed using the Viking software suite.
Results
In this study, Aii ACs from RPC1 were compared to RC1. Initial results indicate no distinct difference in the morphology other than arbor size, which are likely due to eccentricity differences between volumes. However, in RPC1, we observe multiple instances of Aii AC coupling with Rod BCs in the ON region of the IPL. In contrast, Rod BCs never form gap junctions in healthy retina.
Conclusions
Coupling between Aii ACs and Rod BCs in RPC1 is a unique change in retinal network topology occurring in early RD. Further exploration of network changes as a response to RD is warranted, as many therapeutic interventions currently in development rely upon maintenance of inner retinal circuitry. Prior research demonstrates Rod BCs extend dendrites towards cones and change their receptor expression as rods degenerate. Therefore, knowing the network changes involving Aii ACs and their associations with bipolar cells is crucial to understanding how photoreceptor degeneration affects inner retinal visual processing.
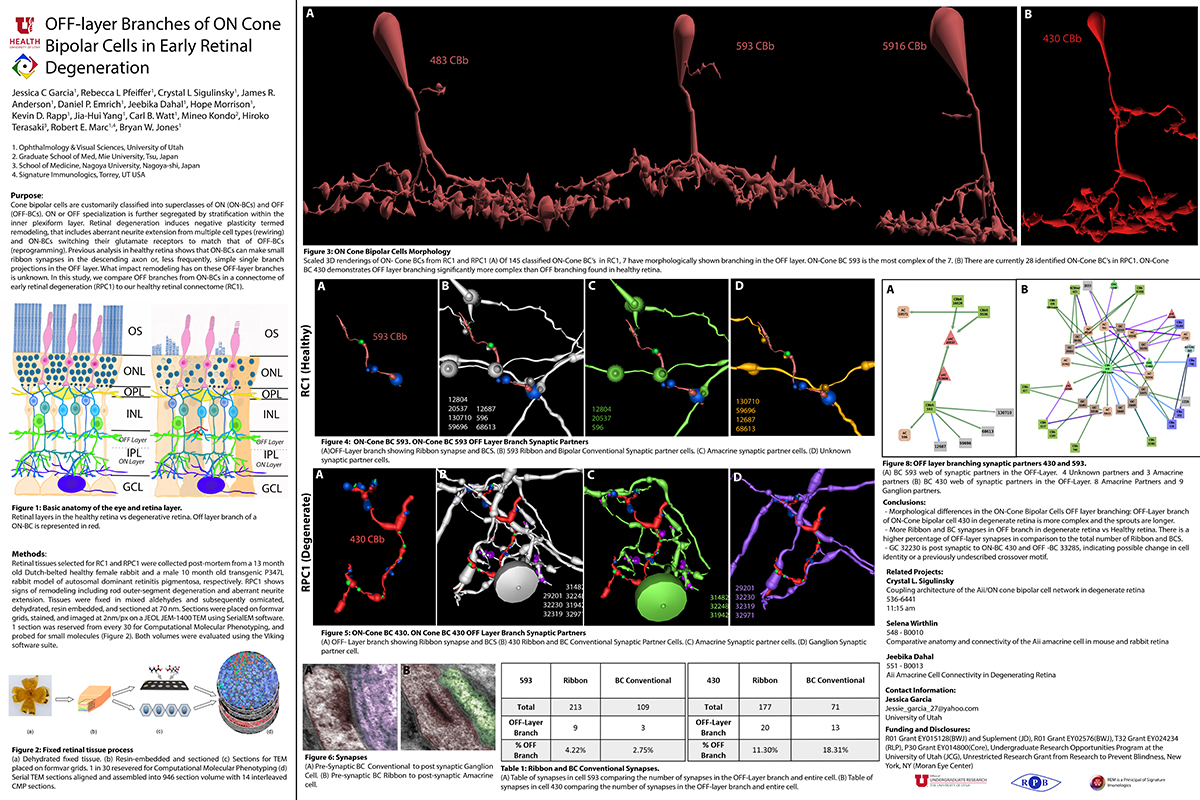
OFF-layer Branches of ON Cone Bipolar Cells in Early Retinal Degeneration
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Jessica C. Garcia, Rebecca L. Pfeiffer, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Jeebika Dahal, Hope Morrison, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Mineo Kondo, Hiroko Terasaki, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose: Cone bipolar cells are customarily classified into superclasses of ON (ON-BCs) and OFF (OFF-BCs). ON or OFF specialization is further segregated by stratification within the inner plexiform layer. Retinal degeneration induces negative plasticity termed remodeling, that includes aberrant neurite extension from multiple cell types (rewiring) and ON-BCs switching their glutamate receptors to match that of OFF-BCs (reprogramming). Previous analysis in healthy retina shows that ON-BCs can make small ribbon synapses in the descending axon or, less frequently, simple single branch projections in the OFF layer. What impact remodeling has on these OFF-layer branches is unknown. In this study, we compare OFF branches from ON-BCs in a connectome of early retinal degeneration (RPC1) to our healthy retinal connectome (RC1).
Methods: Retinal tissues selected for RC1 and RPC1 were collected post-mortem from a 13 month old Dutch-belted healthy female rabbit and a male 10 month old transgenic P347L rabbit model of autosomal dominant retinitis pigmentosa, respectively. RPC1 shows signs of remodeling including rod outer-segment degeneration and aberrant neurite extension. Tissues were fixed in mixed aldehydes and subsequently osmicated, dehydrated, resin embedded, and sectioned at 90 nm (RC1) or 70 nm (RPC1). Sections were placed on formvar grids, stained, and imaged at 2nm/px on a JEOL JEM-1400 TEM using SerialEM software. 1 section was reserved from every 30 for Computational Molecular Phenotyping, and probed for small molecules. Both volumes were evaluated using the Viking software suite.
Results: Ribbons of ON-BCs formed in the OFF layer branches have been previously described to contact glycinergic amacrine cells (ACs), GABAergic ACs, ON ganglion cells, and intrinsically photosensitive ganglion cells. Initial analysis of OFF branches of ON-BCs in RPC1 demonstrate more complex branching than in RC1 and increased number of synapses on these branches. In contrast to the inconsistent OFF layer branch stratification observed in RC1, the OFF branches in RPC1 appear to stratify at a similar level. Evaluation of synaptic partners is ongoing.
Conclusions: Increased complexity and number of synapses found in the OFF branches of some ON-BCs ultimately may represent ON network corruption. Exploring synaptic partners will reveal potential network alterations in retinal degenerative disease.