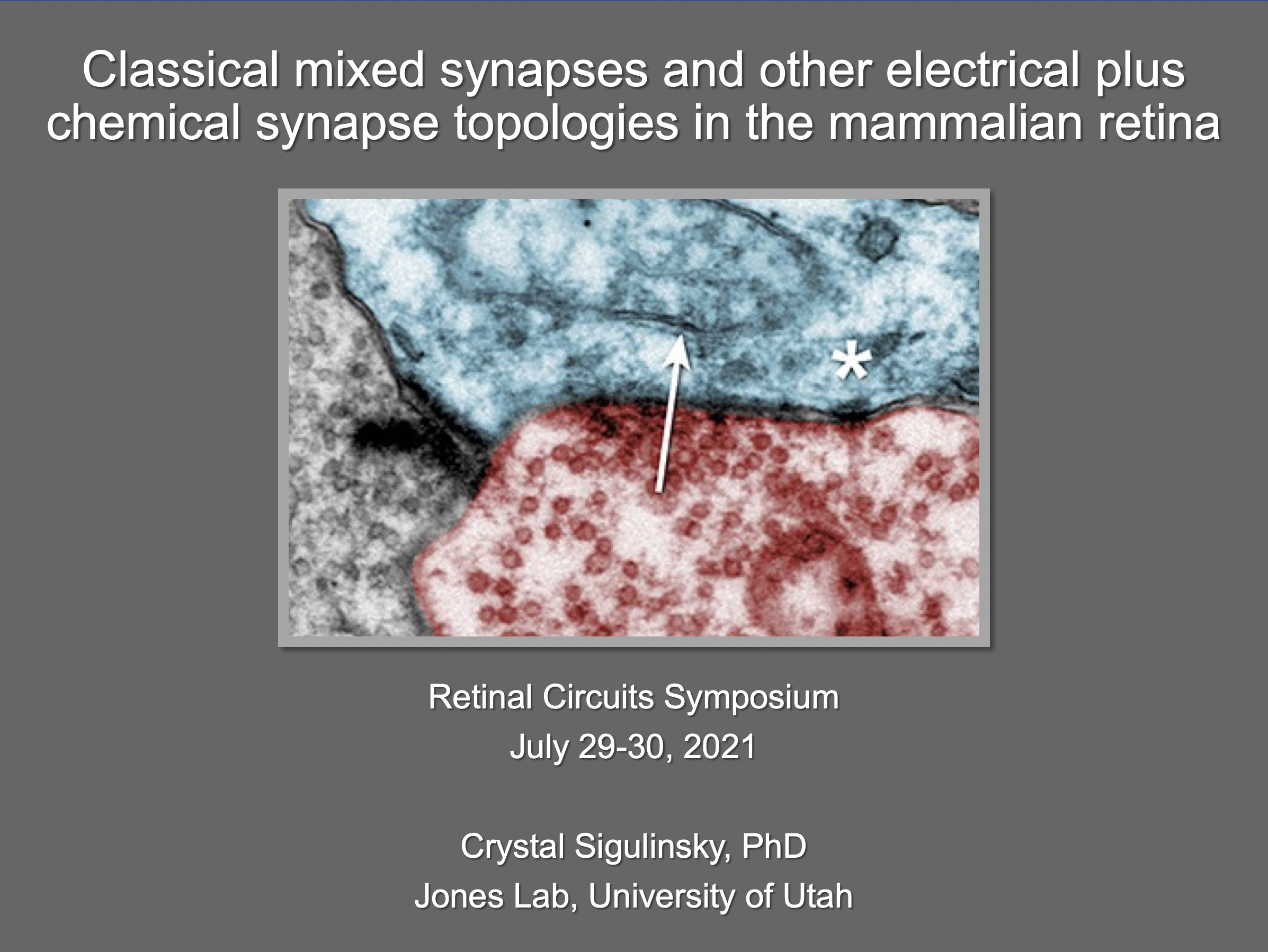
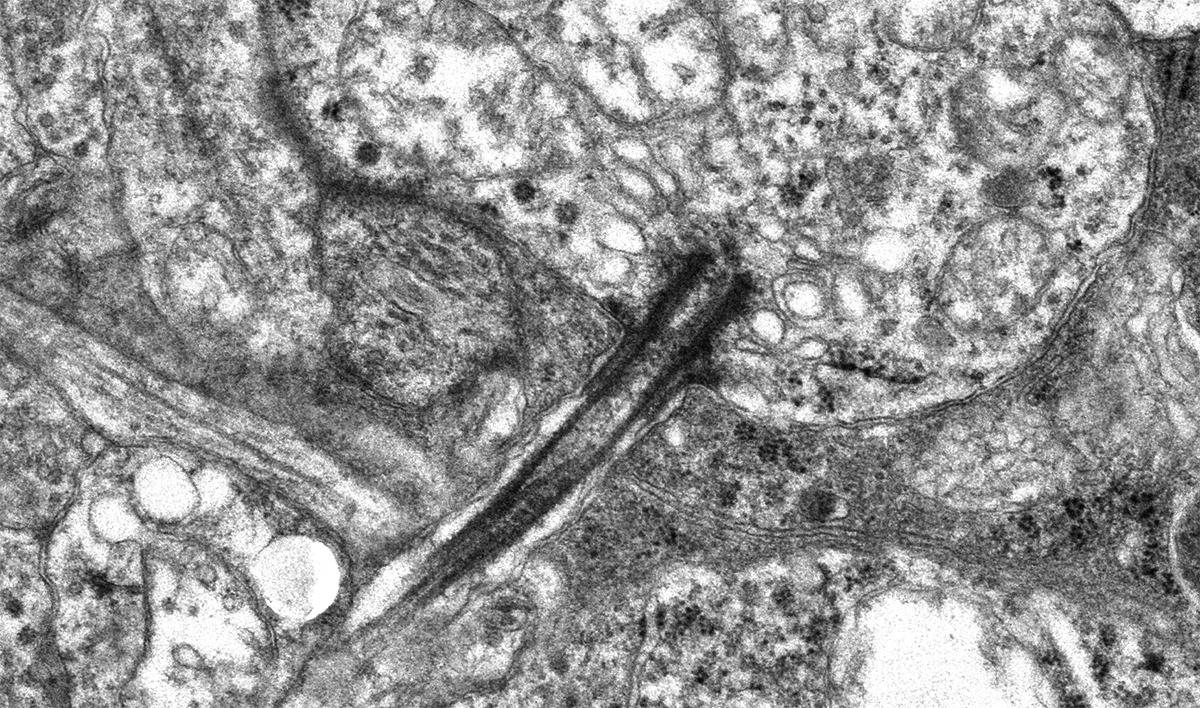
“Those of us who survived, are now through the 2nd year of this pandemic.”
That sentence sounds like the introduction to a post apocalyptic story, and something that while not completely unexpected in my lifetime, still has a rawness to it, and an anger to it because of how the world has handled things. Our distraction with political authoritarianism across the world, and an obsession with individual rights over that of society has made this far worse than it otherwise would in a healthy global society. We are now looking at over 800,000 Americans are dead from COVID, with over 5.4 million dead worldwide, and we are not yet done. If you had told me that these would be the numbers back in the first week of March 2020 when I shut the lab down to protect everyone in it, I might not have believed you.
We started the year 2021 in the lab with a chiller for one of our electron microscopes that failed. That took out half our electron microscopy infrastructure for a period of time, until we could get it back up and running, which of course was complicated by the pandemic. I’m grateful for Mark Kirkpatrick, and Kevin Mcilwrath from JEOL @JEOLUSA who helped us maintain the microscopes and keep them up and running. 2021 continued the hits with lots of new bureaucracy initiatives at the University of Utah with IT, and HR, and purchasing, and travel, that are an incredible tax on time and resources. If I had enough resources to help balance out those demands, it would be acceptable, but as it is, I’m getting further and further away from the science, which is seriously bumming me out. I got into this game to dig deep into the science. This skyrocketing administrative burden on top of all the other things that I’ve had to deal with as a result of the pandemic has really stressed things out. Yet we’ve still been incredibly productive through the pandemic, mostly because of the work of personnel in the lab, Jia-Hui, Jamie, Nat @NatQuayleNelson, Crystal @CSigulinsky, Becca @BeccaPfeiffer19, Jeebika, Selena, Olivia, Isabel and Gabe, all of whom I am immensely grateful for.
This year, despite the 2nd year of a global pandemic, we’ve still managed to publish a book chapter, 6 manuscripts, 2 abstracts, participated in teaching new faculty at the University of Utah School of Medicine, co-organized the Moran Eye Center Translational Research Day with Leah Owen, started faculty hiring initiatives, reviewed grants, started a new retinal connectomics initiative that will meet for the first time in January, submitted a grant renewal (triaged), submitted three other grants (not funded), taught classes for grad students, started serving on 3 PhD student committees, brought a couple of new undergraduates into the lab, had one undergraduate win Outstanding Undergraduate Researcher of The Year Award, had postdocs present at a couple of meetings, had a couple of PhD students rotate through the lab, gave 5 invited talks, initiated two more collaborations and more, all while struggling through unprecedented difficulties related to the pandemic.
The New Year 2022 looks promising, and we hope for a slow normalization of things. January will bring a new technician to the lab, and the first meeting of a select group of people for a new retinal connectomics initiative that we’ll be rolling out to a wider audience ASAP. We’ll also have a new collaborative manuscript that has just been accepted in Nature. Later in the first part of the year, we’ll have another three manuscripts submitted, a grant renewal that will go in, and we might have a new PhD student joining the lab as well as a new undergraduate. Hopefully later in the year, some of the irons that I have in the fire will return on those investments, and we’ll start to normalize some other things like meetings. ARVO is planning on being a hybrid meeting, and I’ve agreed to a special interest group presentation there with Michael Redmond. We’ll see what other meetings happen next year, pending outcomes from the pandemic. I’d love to be able to send the postdocs to meetings this year. ISER was pushed out to February of 2023 because of the pandemic, so that won’t be happening this year as was previously planned. Further out in the year is harder to predict, but we should be finished with our third pathoconnectome, as well as our primate retinal connectome, paving the way for us to begin building our two human pathoconnectomes.
However this year ends up, we here at the Marclab for Connectomics wish you a Happy New Year for 2022, and much success and happiness.