We have a new preprint out, Neural Circuit Revision in Retinal Remodeling, A Pathoconnectomics Approach.
Authors: Rebecca L Pfeiffer, Jeebika Dahal, Crystal L Sigulinsky, James R Anderson, Isabel A Barrera, Jia-Hui Yang, Olivia Haddadin, Alexis R Houser, Jessica C Garcia, Bryan William Jones
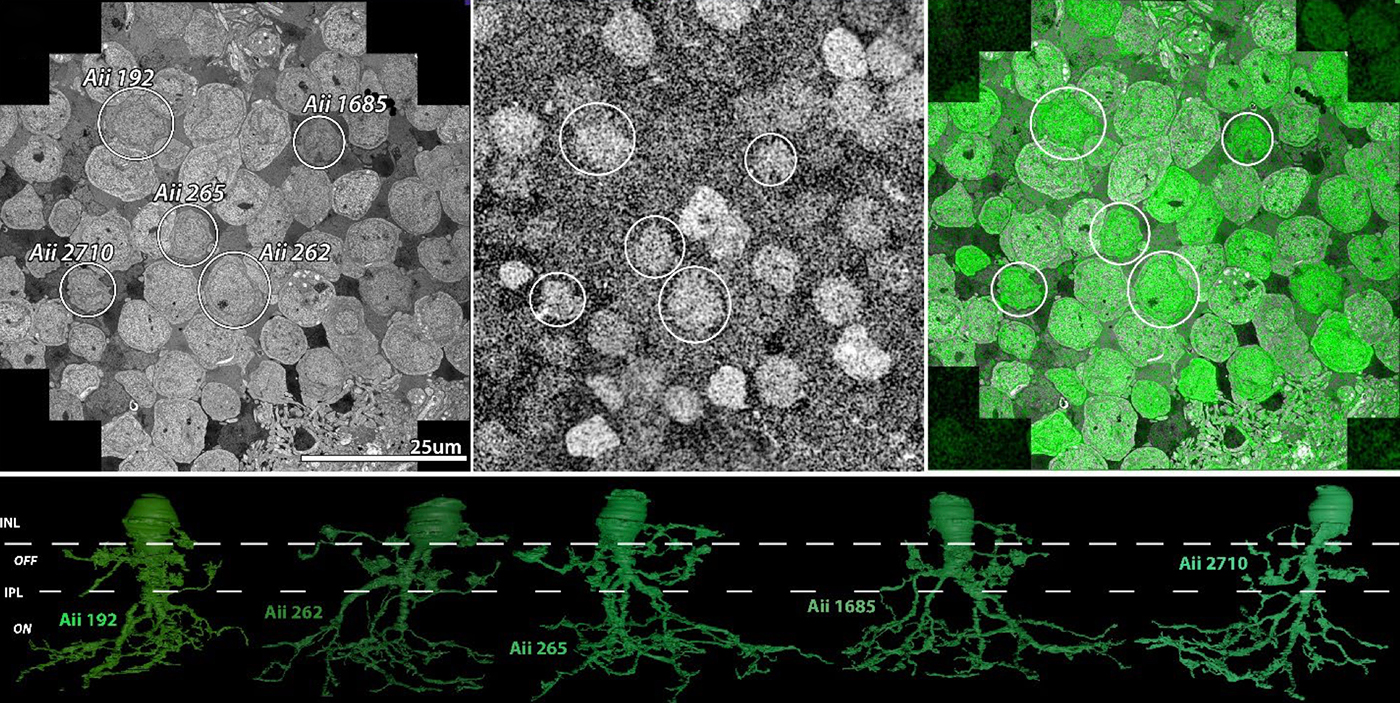
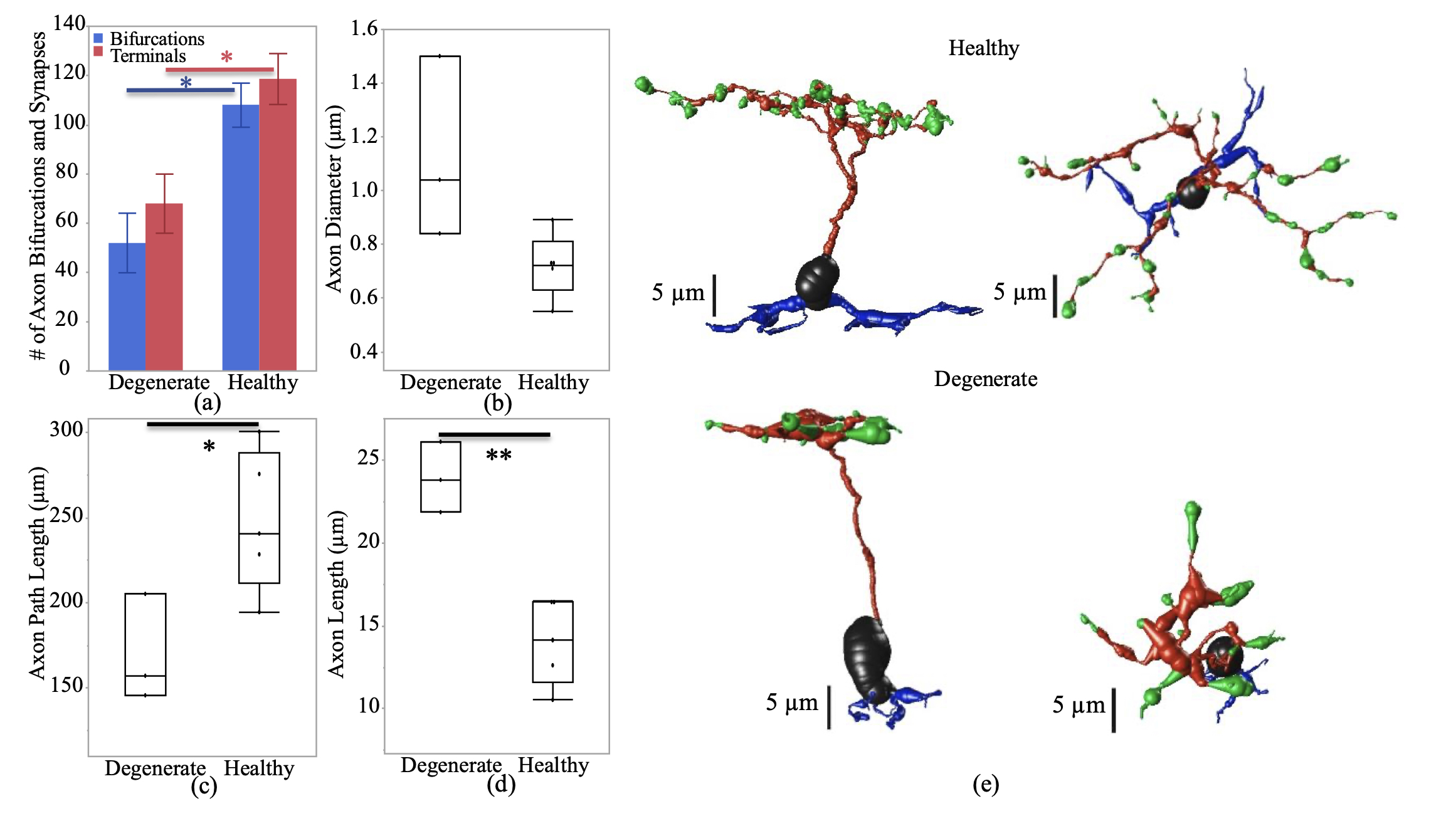
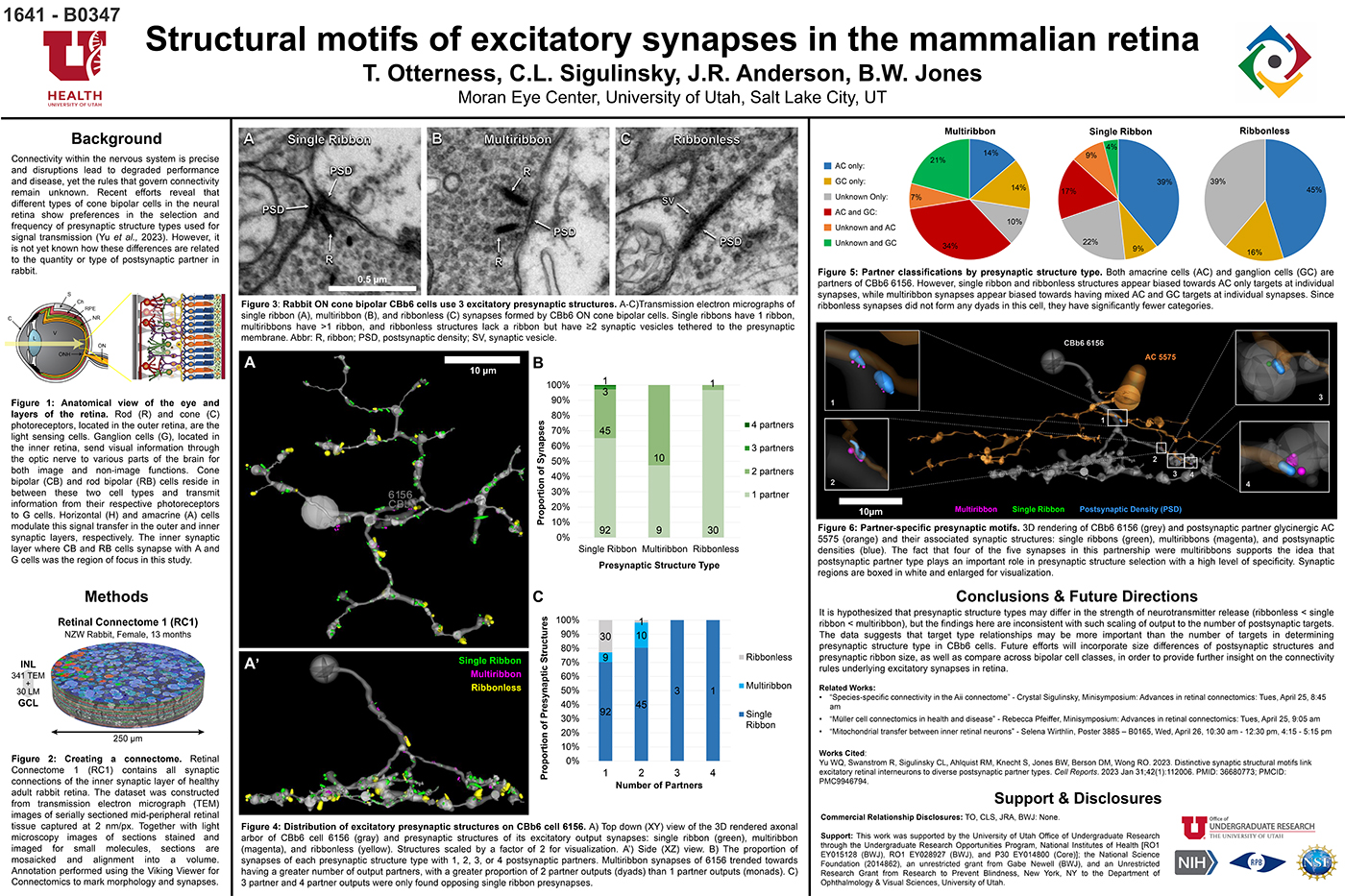
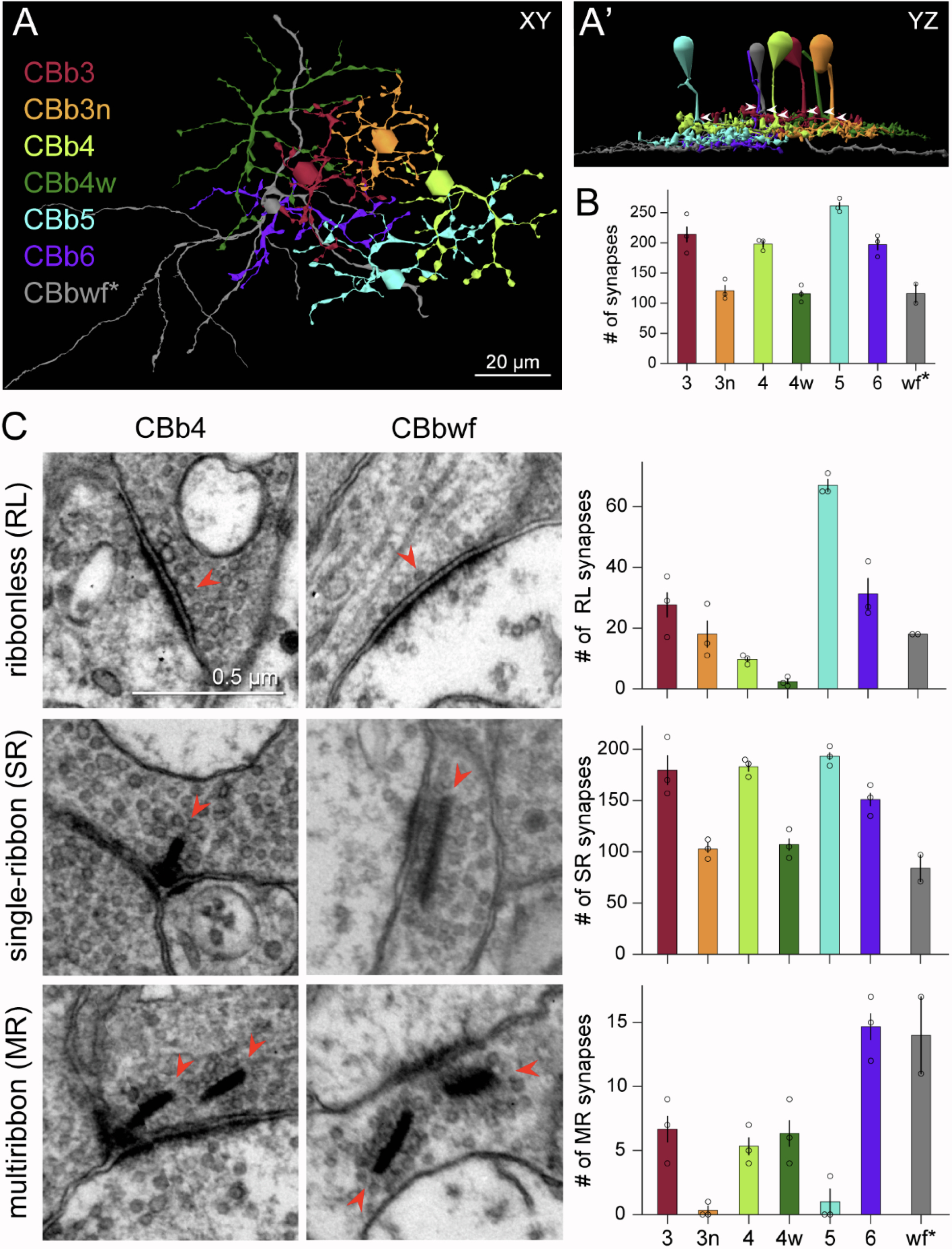
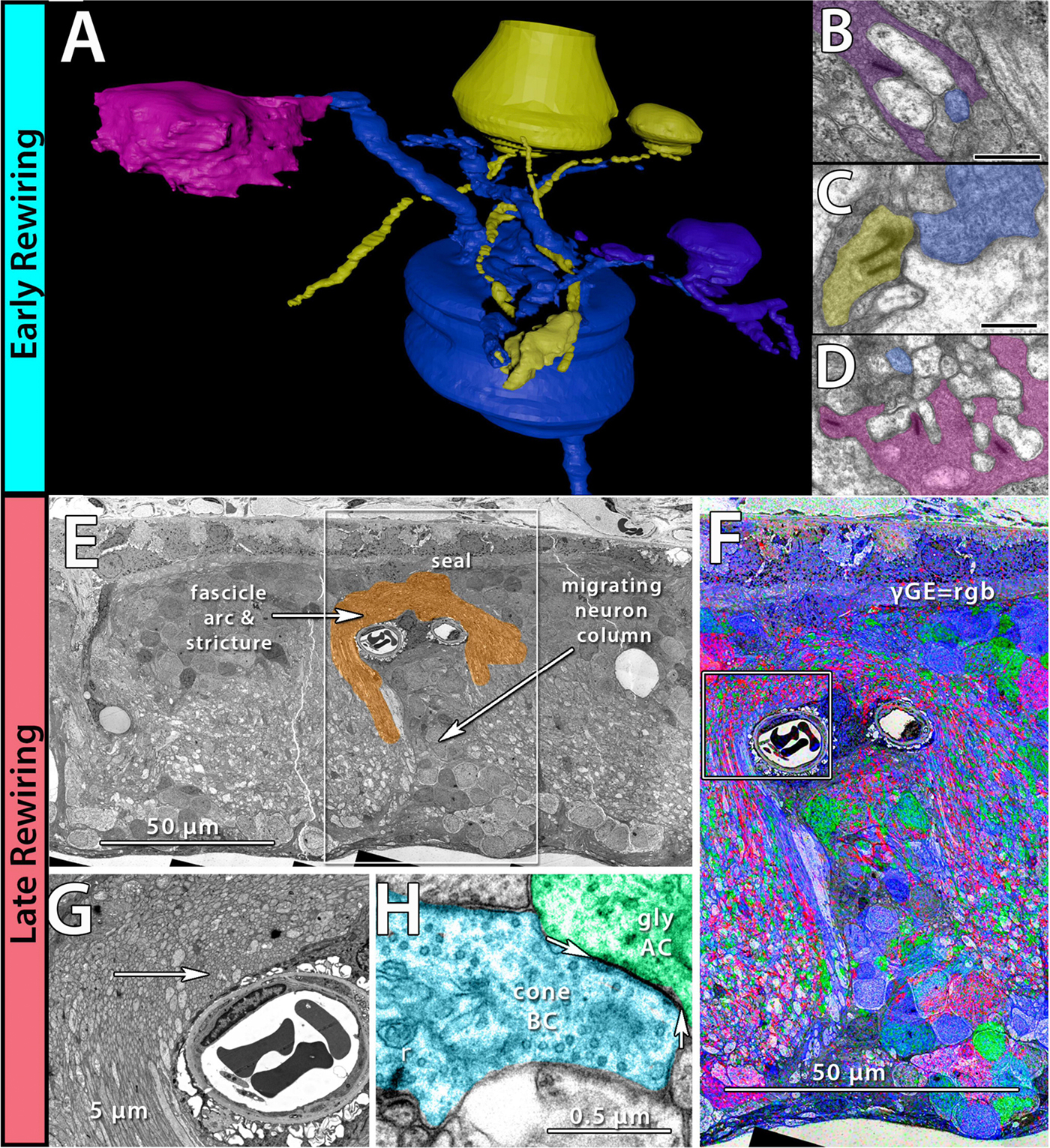
Abstract: The Aii glycinergic amacrine cell (Aii) plays a central role in bridging rod pathways with cone pathways, enabling an increased dynamic range of vision from scotopic to photopic ranges. The Aii integrates scotopic signals via chemical synapses from rod bipolar cells (RodBCs) onto the arboreal processes of Aii ACs, injecting signals into ON-cone bipolar cells (CBbs) via gap junctions with Aiis on the arboreal processes and the waist of the Aii ACs. The CBbs then carry this information to ON and OFF ganglion cell classes. In addition, the Aii is involved in the surround inhibition of OFF cone bipolar cells (CBas) through glycinergic chemical synapses from Aii ACs onto CBas. We have previously shown changes in RodBC connectivity as a consequence of rod photoreceptor degeneration in a pathoconnectome of early retinal degeneration: RPC1. Here, we evaluated the impact of rod photoreceptor degeneration on the connectivity of the Aii to determine the impacts of photoreceptor degeneration on the downstream network of the neural retina and its suitability for integrating therapeutic interventions as rod photoreceptors are lost. Previously, we reported that in early retinal degeneration, prior to photoreceptor cell loss, Rod BCs make pathological gap junctions with Aiis. Here, we further characterize this altered connectivity and additional shifts in both the excitatory drive and gap junctional coupling of Aiis in retinal degeneration, along with discussion of the broader impact of altered connectivity networks. New findings reported here demonstrate that Aiis make additional gap junctions with CBas increasing the number of BC classes that make pathological gap junctional connectivity with Aiis in degenerating retina. In this study, we also report that the Aii, a tertiary retinal neuron alters their synaptic contacts early in photoreceptor degeneration, indicating that rewiring occurs in more distant members of the retinal network earlier in degeneration than was previously predicted. This rewiring impacts retinal processing, presumably acuity, and ultimately its ability to support therapeutics designed to restore image-forming vision. Finally, these Aii alterations may be the cellular network level finding that explains one of the first clinical complaints from human patients with retinal degenerative disease, an inability to adapt back and forth from photopic to scotopic conditions.