We have a new publication out (direct link), Increasing Electrical Stimulation Efficacy in Degenerated Retina: Stimulus Waveform Design in a Multiscale Computational Model authored by Kyle Loizos, Robert Marc, Mark Humayun, James R. Anderson, Bryan W. Jones and Gianluca Lazzi.
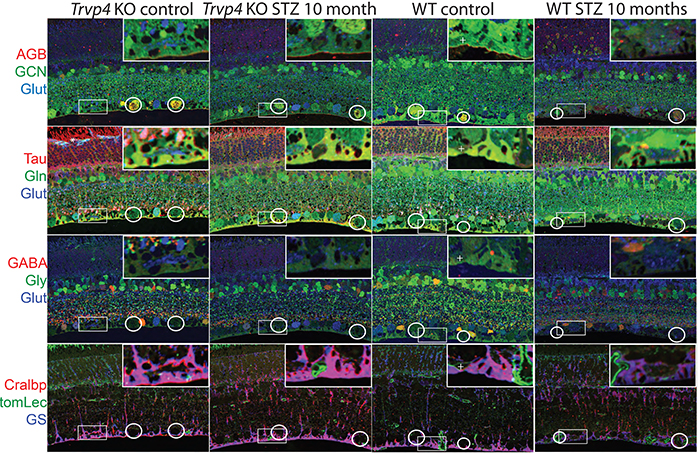
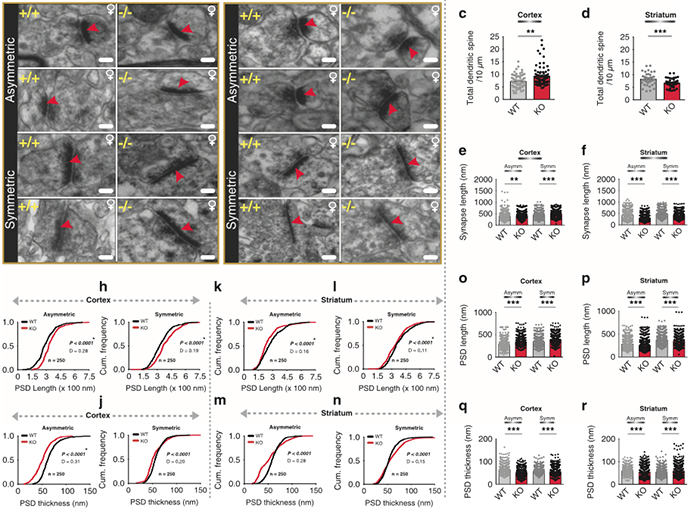
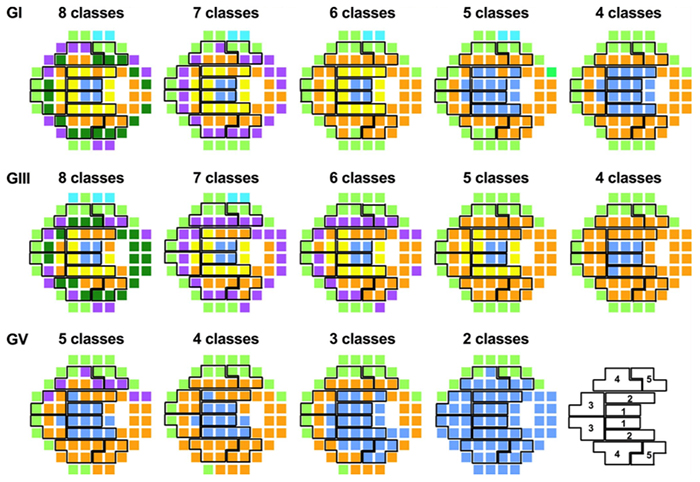
Abstract—A computational model of electrical stimulation of the retina is proposed for investigating current waveforms used in prosthetic devices for restoring partial vision lost to retina degen- erative diseases. The model framework combines a connectome- based neural network model characterized by accurate mor- phological and synaptic properties with an Admittance Method model of bulk tissue and prosthetic electronics. In this model, the retina was computationally “degenerated,” considering cellular death and anatomical changes that occur early in disease, as well as altered neural behavior that develops throughout the neurodegeneration and is likely interfering with current attempts at restoring vision. A resulting analysis of stimulation range and threshold of ON ganglion cells within retina that are either healthy or in beginning stages of degeneration is presented for currently-used stimulation waveforms, and an asymmetric biphasic current stimulation for subduing spontaneous firing to allow increased control over ganglion cell firing patterns in degenerated retina is proposed. Results show that stimulation thresholds of retinal ganglion cells do not notably vary after beginning stages of retina degeneration. In addition, simulation of proposed asymmetric waveforms showed the ability to enhance the control of ganglion cell firing via electrical stimulation.