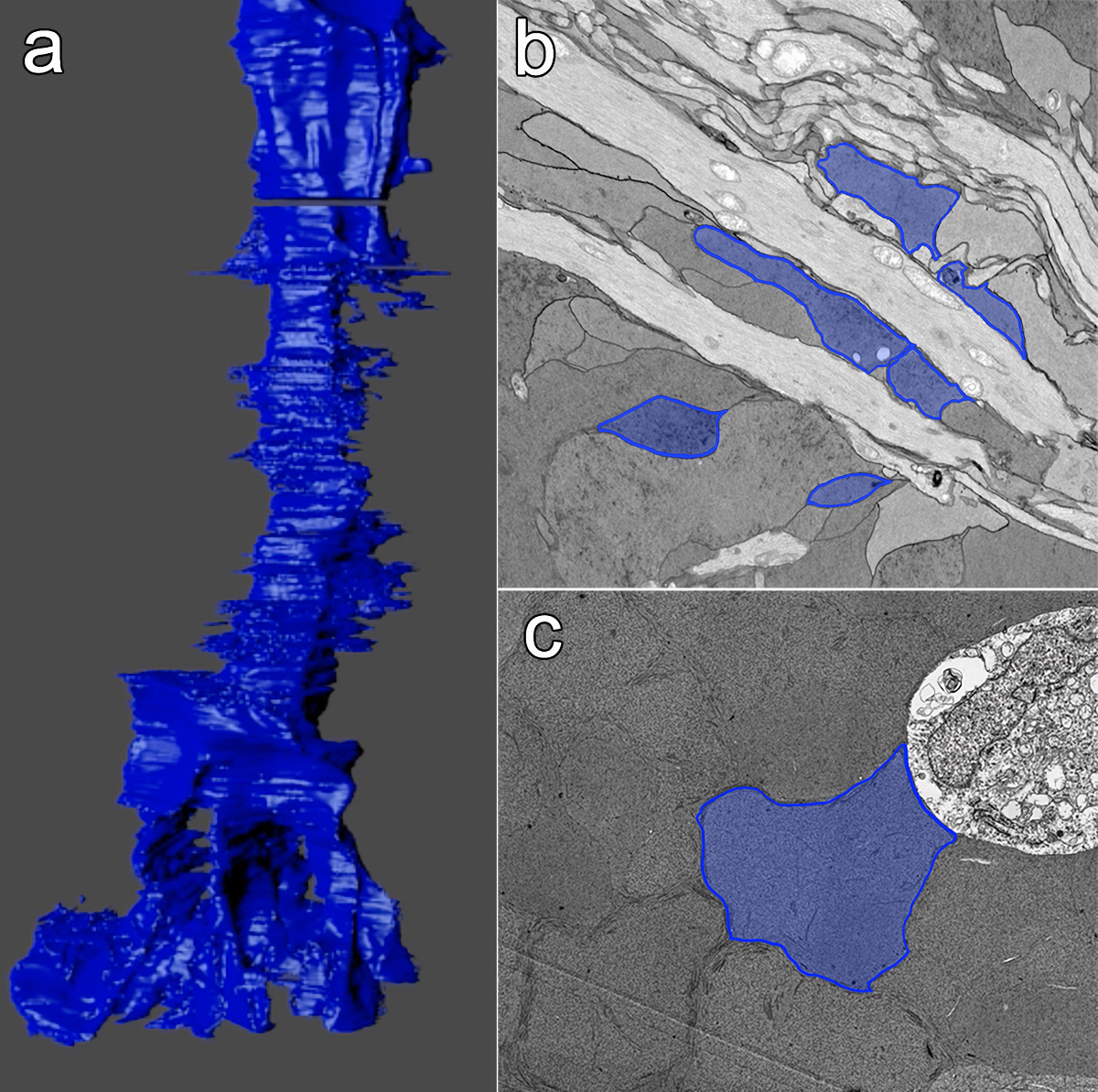
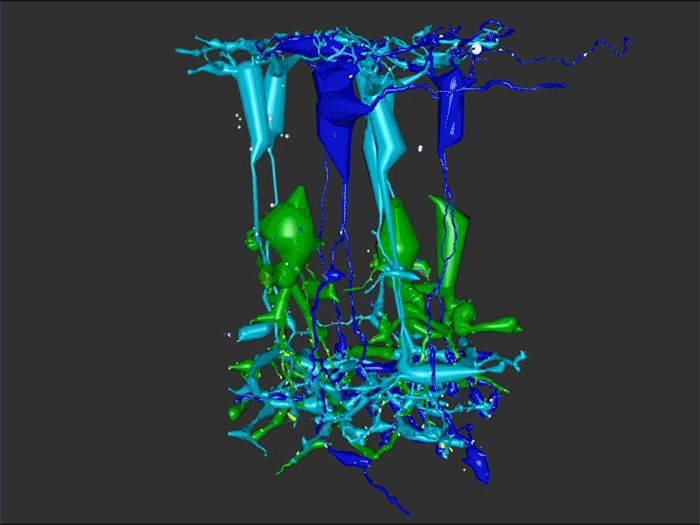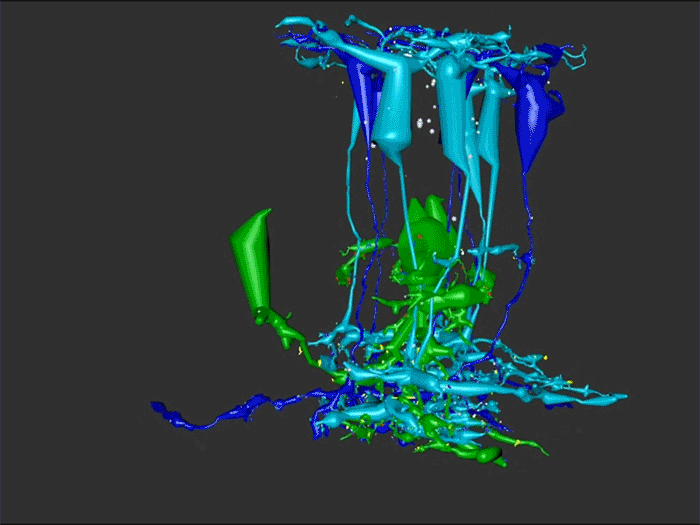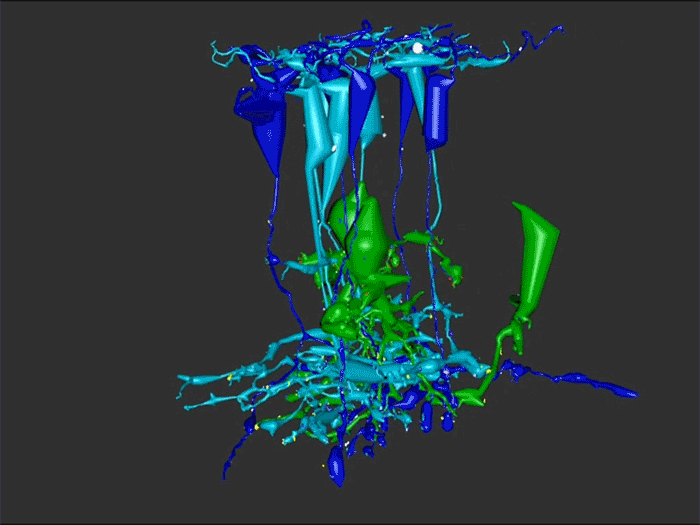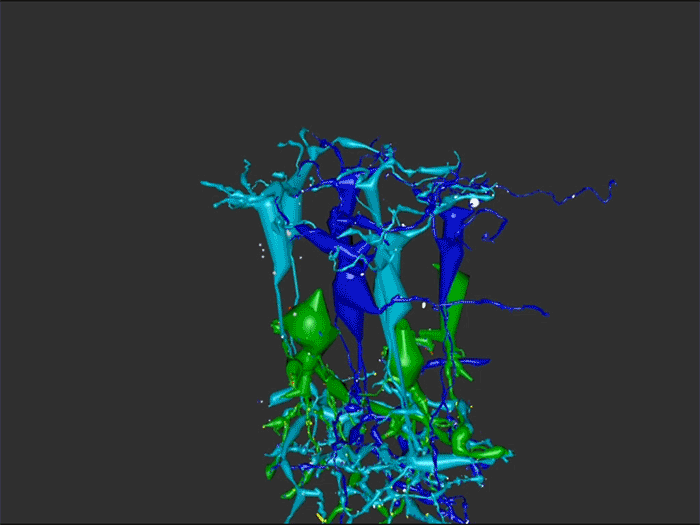
We have a new manuscript out in The Journal of Neuroscience, Network Architecture of Gap Junctional Coupling among Parallel Processing Channels in the Mammalian Retina.
Authors: Crystal L. Sigulinsky @CLSigulinsky, James R. Anderson, Ethan Kerzner @EthanKerzner, Christopher N. Rapp @ChrisNRapp, Rebecca L. Pfeiffer @BeccaPfeiffer19, Taryn M. Rodman, Daniel P. Emrich, Kevin D. Rapp, Noah T. Nelson @nooneelseinhere, J. Scott Lauritzen, Miriah Meyer@miriah_meyer, Robert E. Marc @robertmarc60, and Bryan W. Jones @BWJones.
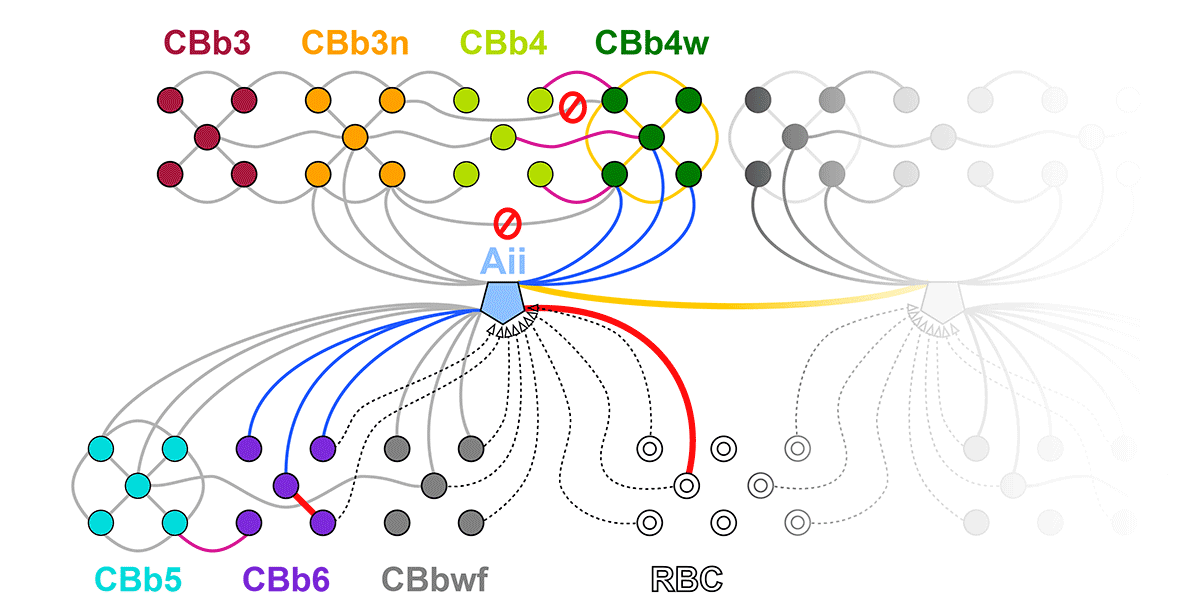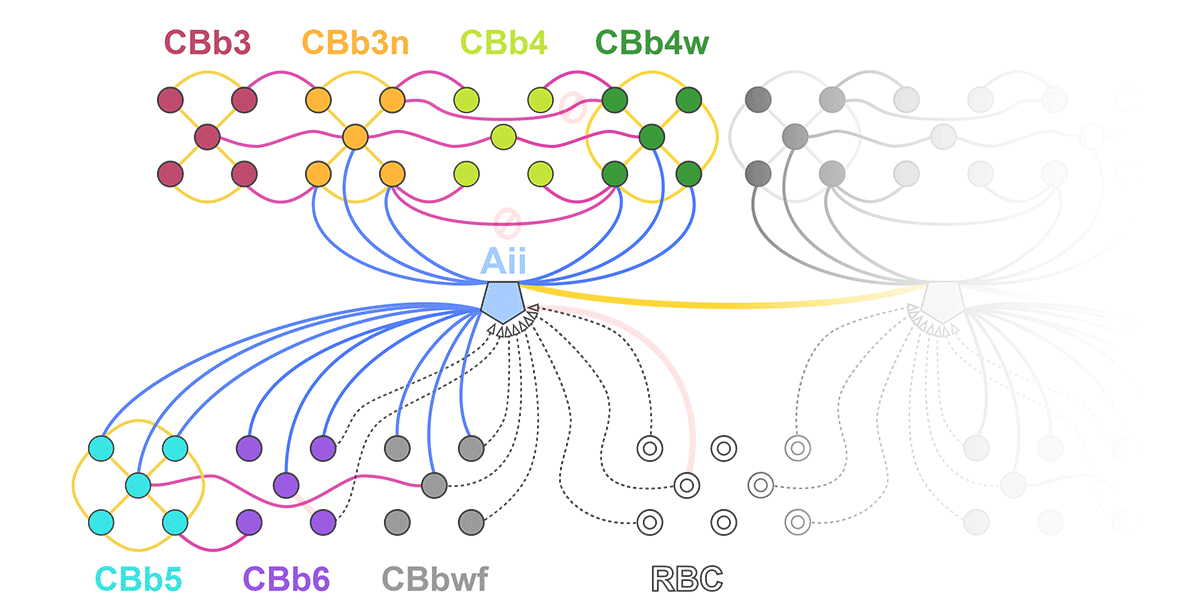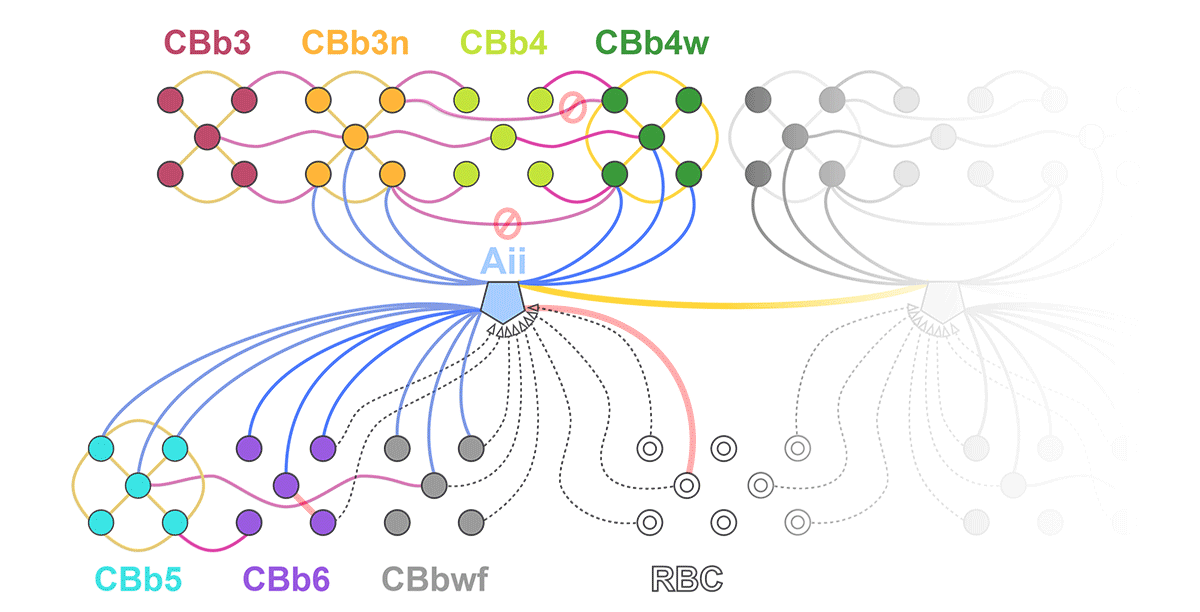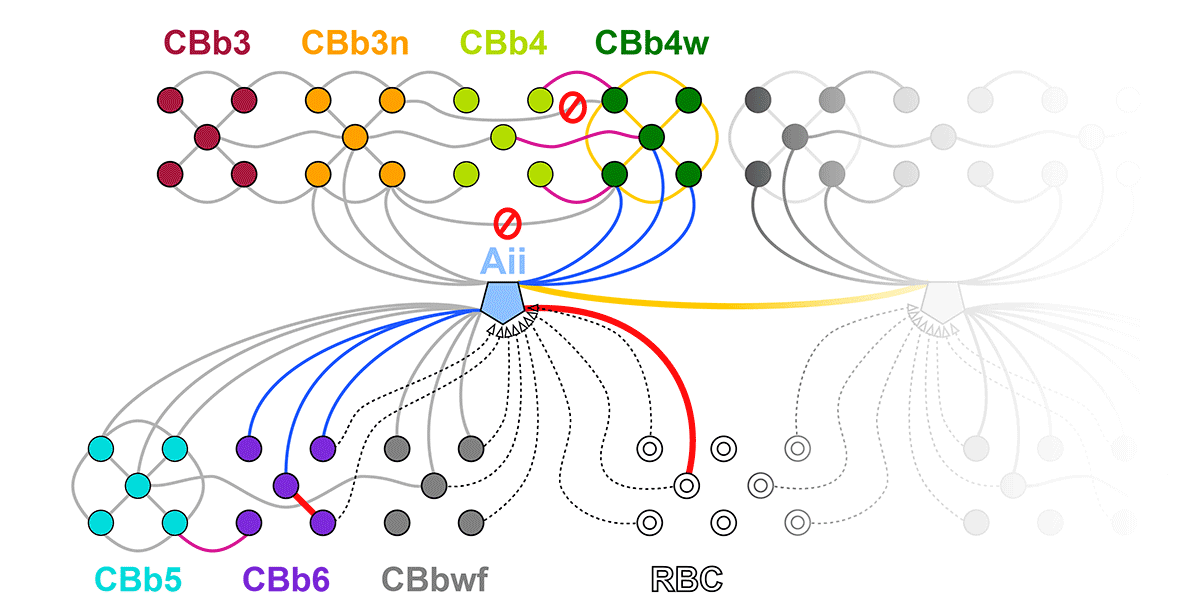
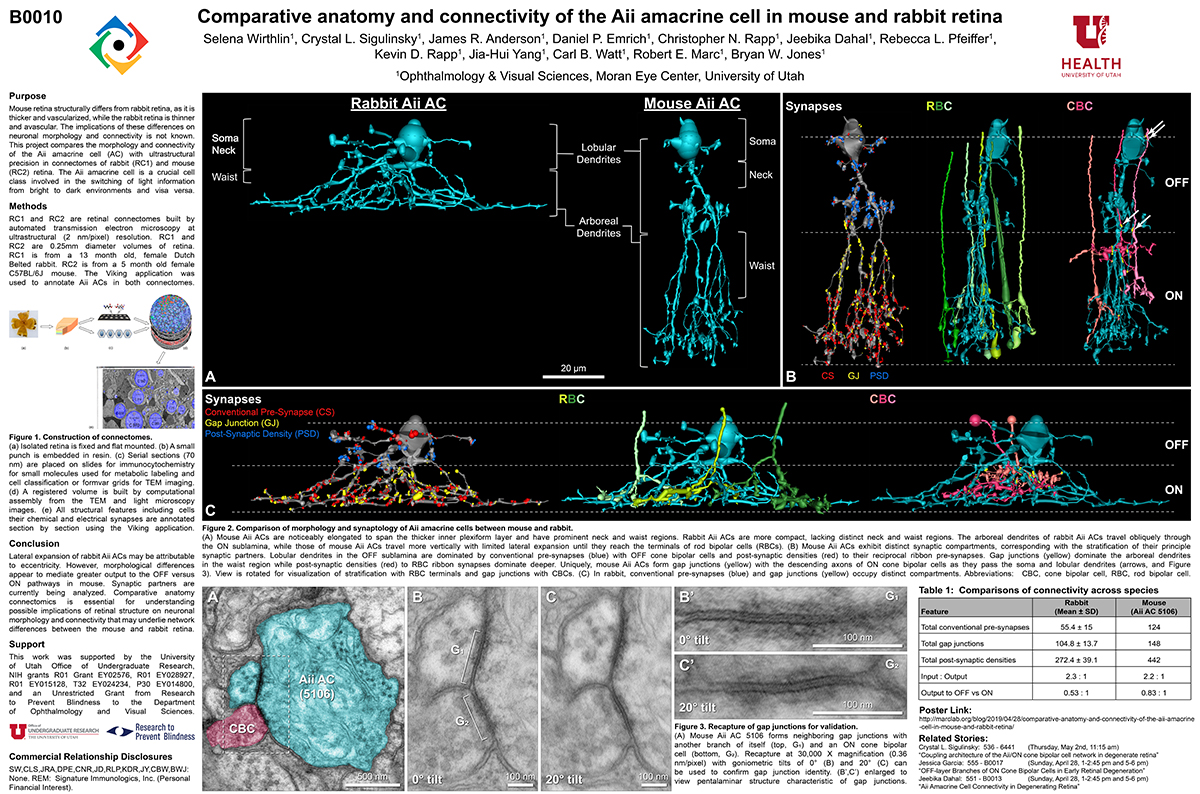
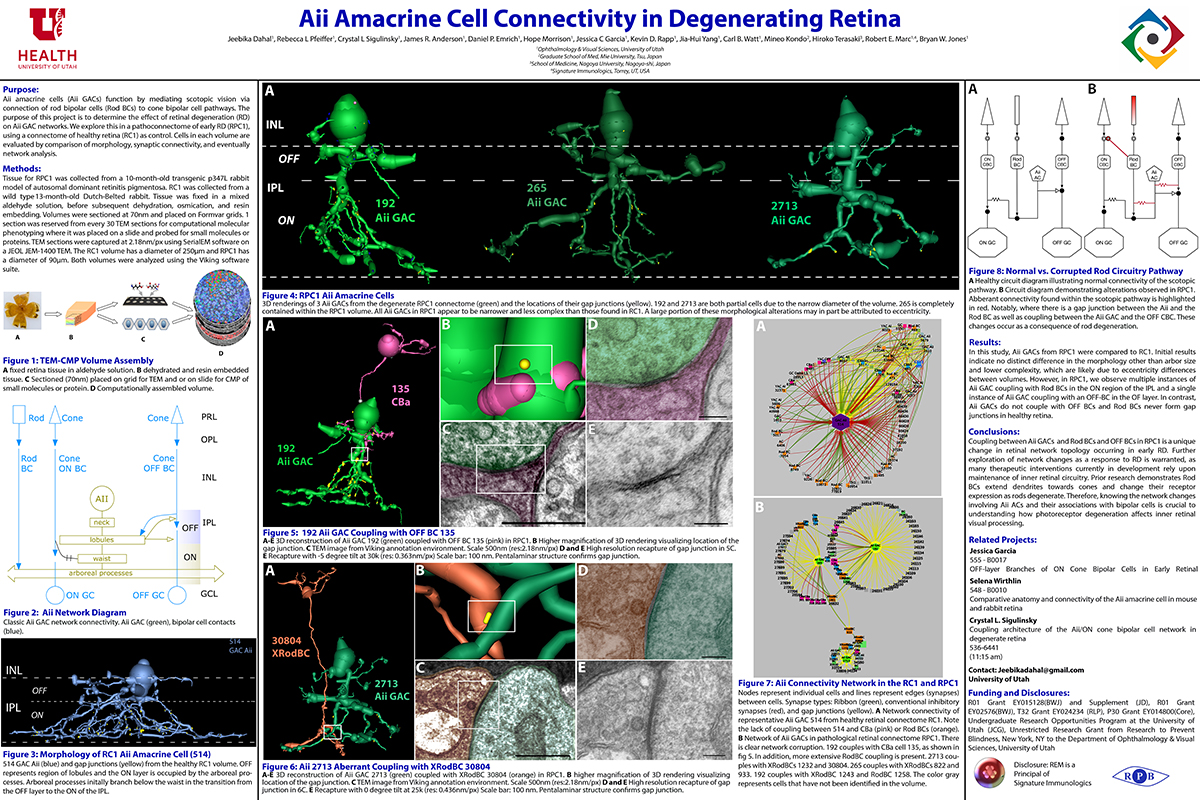
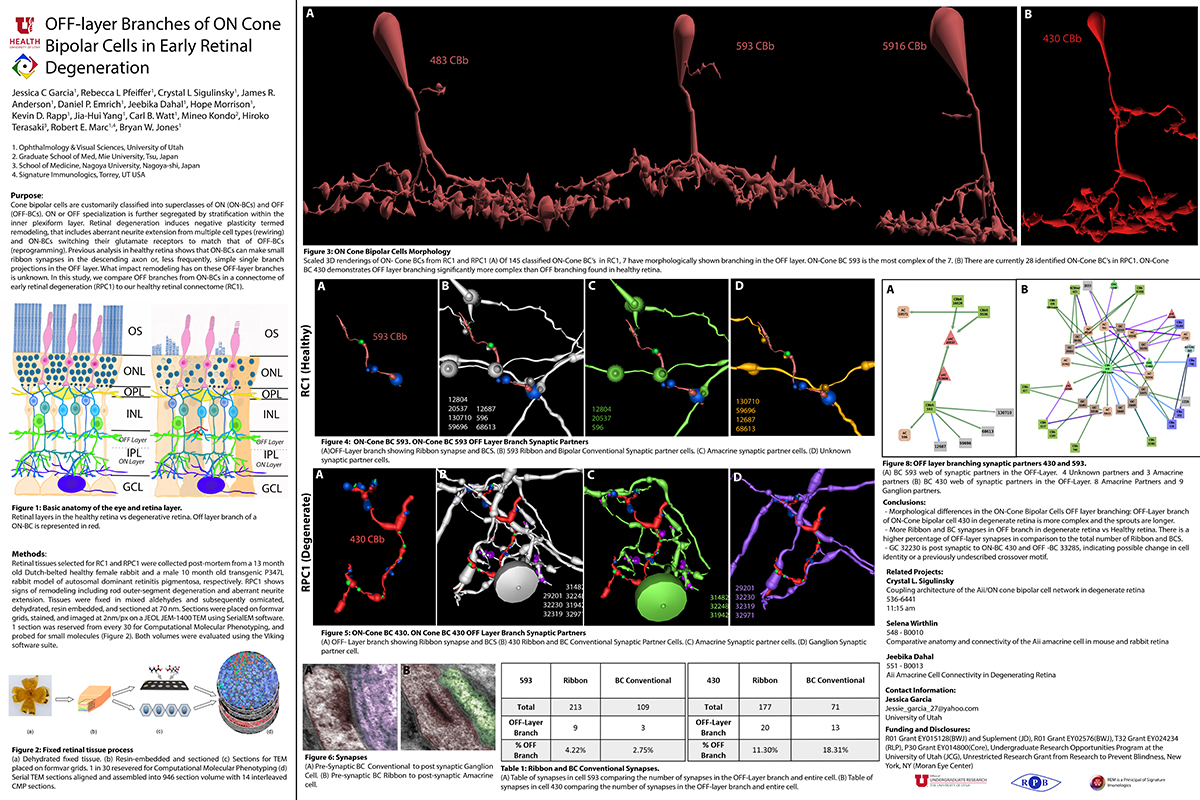
Abstract: Gap junctions are ubiquitous throughout the nervous system, mediating critical signal transmission and integration, as well as emergent network properties. In mammalian retina, gap junctions within the Aii amacrine cell-ON cone bipolar cell (CBC) network are essential for night vision, modulation of day vision, and contribute to visual impairment in retinal degenerations, yet neither the extended network topology nor its conservation is well established. Here, we map the network contribution of gap junctions using a high-resolution connectomics dataset of an adult female rabbit retina. Gap junctions are prominent synaptic components of ON CBC classes, constituting 5%–25% of all axonal synaptic contacts. Many of these mediate canonical transfer of rod signals from Aii cells to ON CBCs for night vision, and we find that the uneven distribution of Aii signals to ON CBCs is conserved in rabbit, including one class entirely lacking direct Aii coupling. However, the majority of gap junctions formed by ON CBCs unexpectedly occur between ON CBCs, rather than with Aii cells. Such coupling is extensive, creating an interconnected network with numerous lateral paths both within, and particularly across, these parallel processing streams. Coupling patterns are precise with ON CBCs accepting and rejecting unique combinations of partnerships according to robust rulesets. Coupling specificity extends to both size and spatial topologies, thereby rivaling the synaptic specificity of chemical synapses. These ON CBC coupling motifs dramatically extend the coupled Aii-ON CBC network, with implications for signal flow in both scotopic and photopic retinal networks during visual processing and disease.