We have a new publication out (direct link), The rod-cone crossover connectome of mammalian bipolar cells authored by Scott Lauritzen, Crystal Sigulinsky, James Anderson, Michael Kalloniatis, Noah Nelson, Danny Emrich, Chris Rapp, Nicolas McCarthy, Ethan Kerzner, Mariah Meyer, Bryan W. Jones, and Robert Marc.
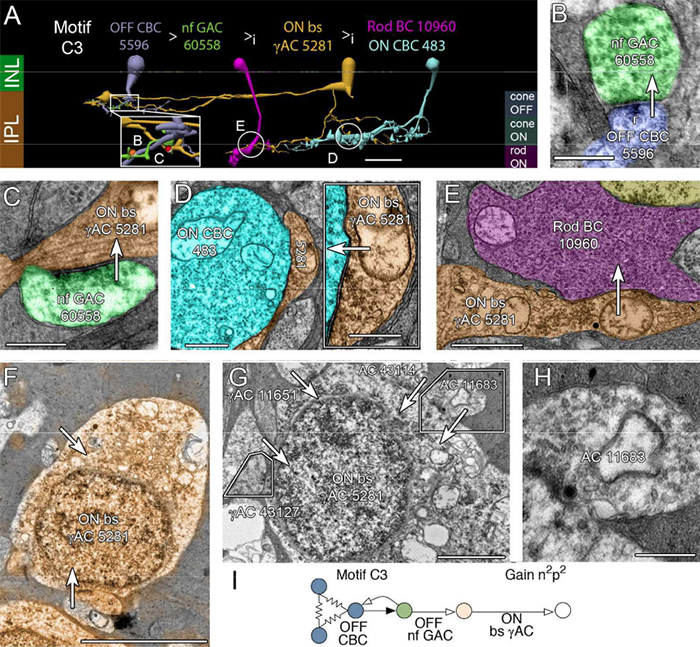
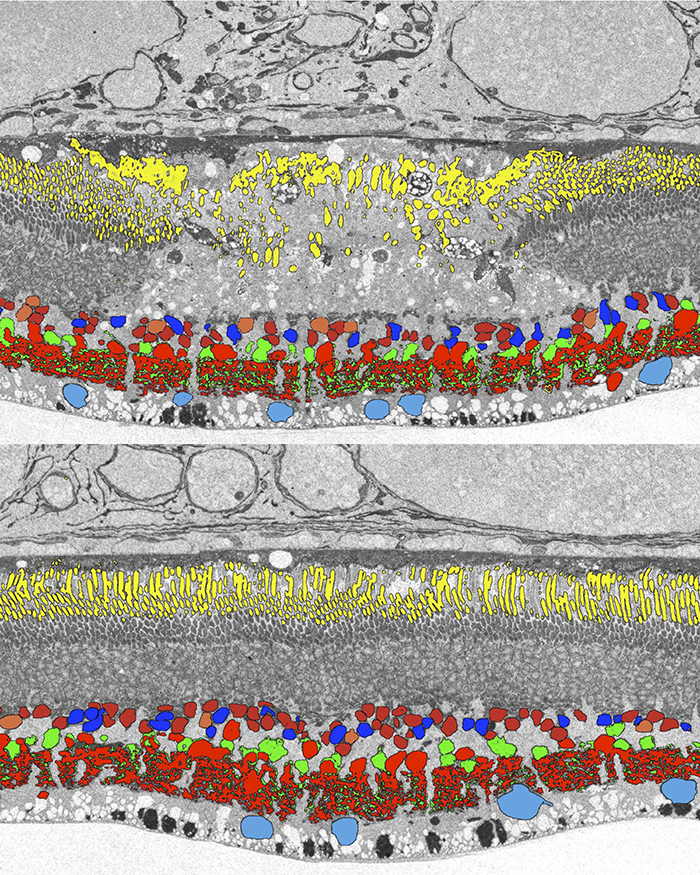
Abstract: The basis of cross-suppression between rod and cone channels has long been an enigma. Using rabbit retinal connectome RC1, we show that all cone bipolar cell (BC) classes inhibit rod BCs via amacrine cell (AC) motifs (C1-6); that all cone BC classes are themselves inhibited by AC motifs (R1-5, R25) driven by rod BCs. A sparse symmetric AC motif (CR) is presynaptic and postsynaptic to both rod and cone BCs. ON cone BCs of all classes drive inhibition of rod BCs via motif C1 wide-field GABAergic ACs (γACs) and motif C2 narrow field glycinergic ON ACs (GACs). Each rod BC receives ≈ 10 crossover AC synapses and each ON cone BC can target ≈ 10 or more rod BCs via separate AC processes. OFF cone BCs mediate monosynaptic inhibition of rod BCs via motif C3 driven by OFF γACs and GACs and disynaptic inhibition via motifs C4 and C5 driven by OFF wide-field γACs and narrow-field GACs, respectively. Motifs C4 and C5 form halos of 60-100 inhibitory synapses on proximal dendrites of AI γACs. Rod BCs inhibit surrounding arrays of cone BCs through AII GAC networks that access ON and OFF cone BC patches via motifs R1, R2, R4 R5 and a unique ON AC motif R3 that collects rod BC inputs and targets ON cone BCs. Crossover synapses for motifs C1, C4, C5 and R3 are 3-4x larger than typical feedback synapses, which may be a signature for synaptic winner-take-all switches.