This talk was presented today, April 25th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Rebecca Pfeiffer as part of an ARVO Minisymposium Bryan William Jones organized.
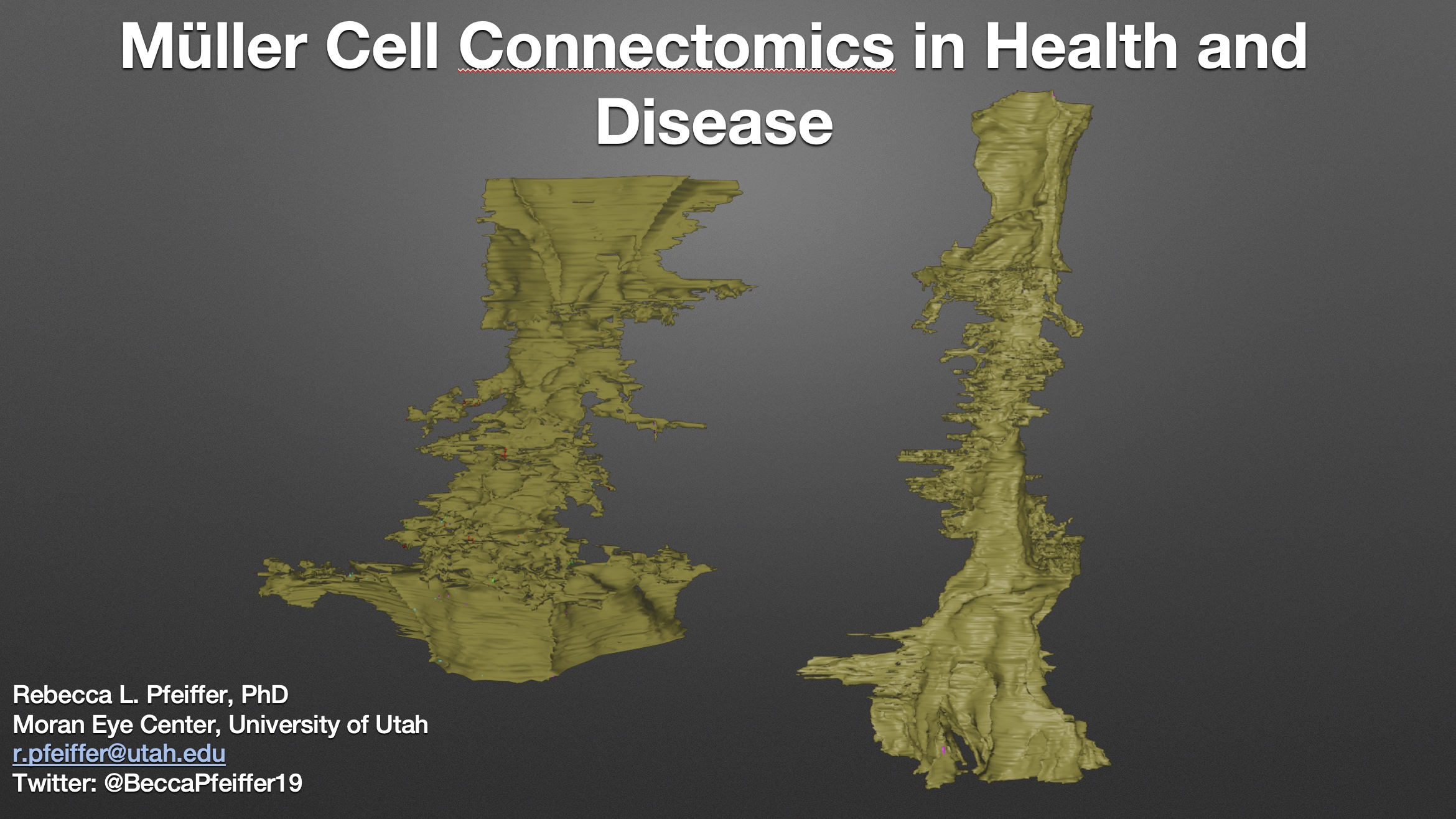
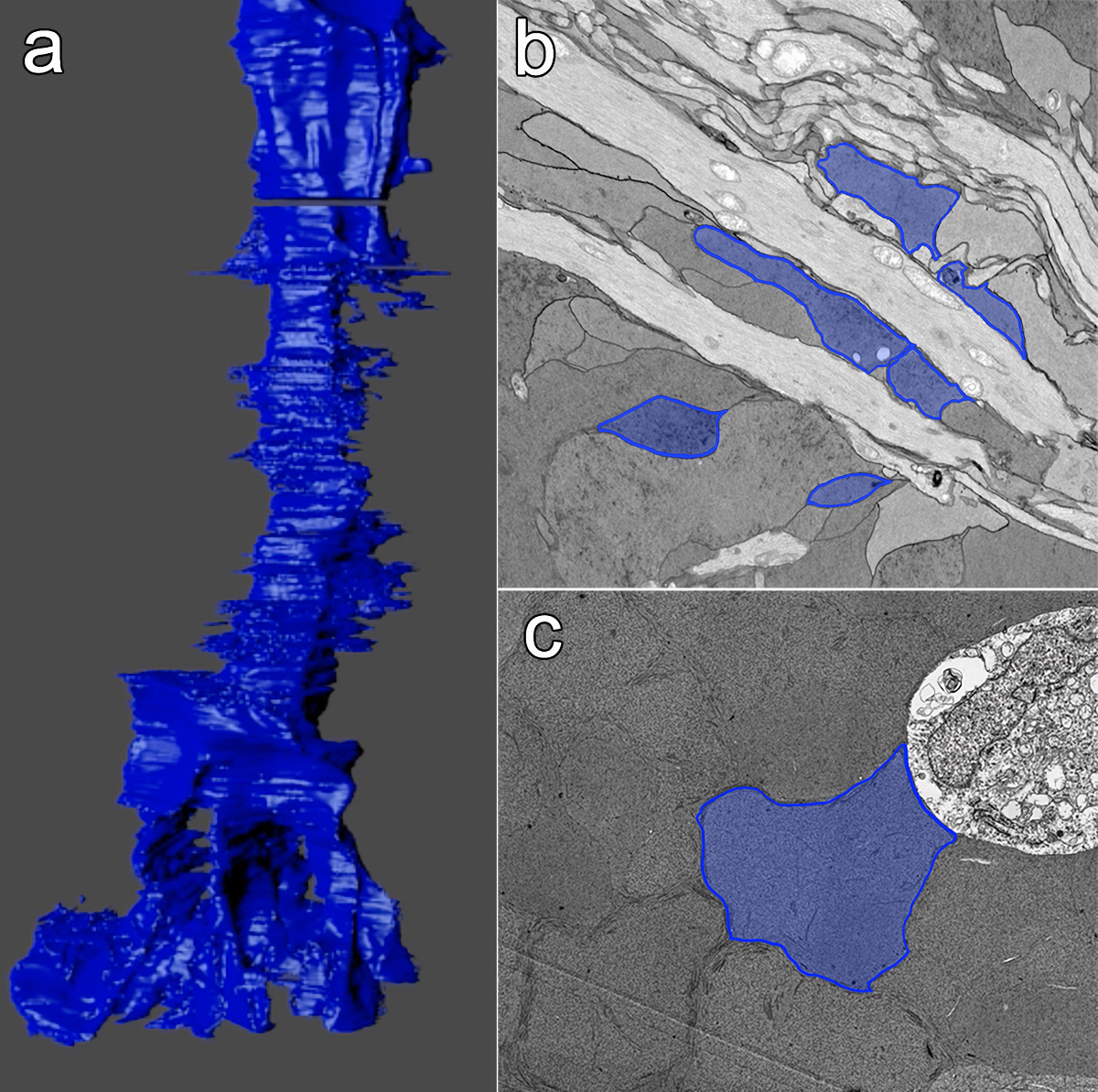
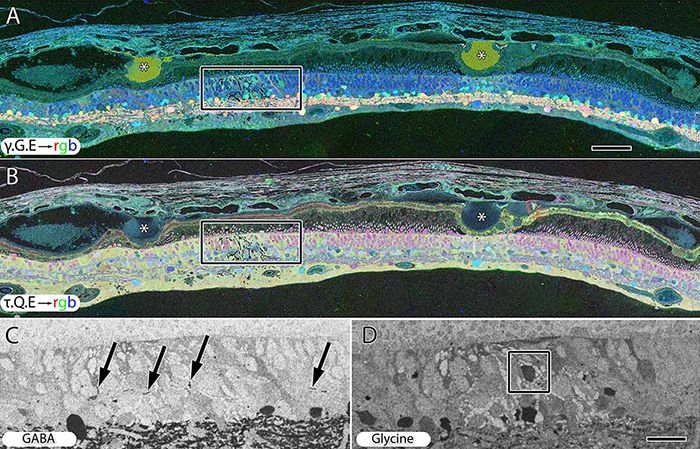
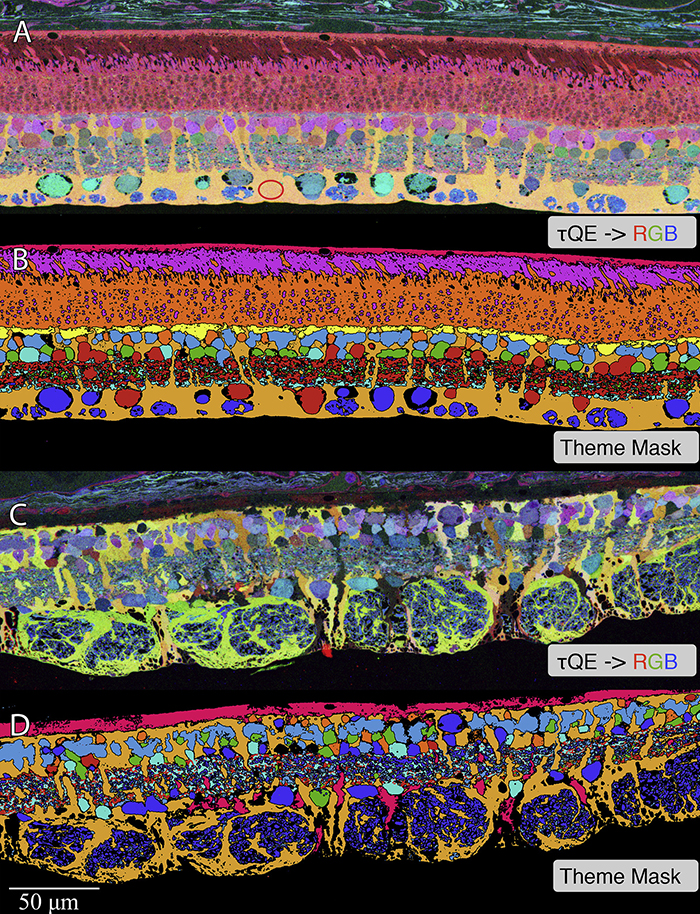
Abstract: Muller cells are a critical component of retinal function and rapidly change metabolically and morphologically in retinal disease. Of Muller cell functions, many require close physical relationships between the Muller cell and the synapses of the neurons they support. Despite this required neuro-glial relationship, little is known about the direct contacts between Muller cells and synapses in healthy or diseased retinas. In order to address this, I use a connectomics/pathoconnectomics approach to reconstruct Muller cells and their neighboring synapses. The retinas evaluated are from a healthy rabbit, retinal connectome 1 (RC1), and from the P347L rabbit model of retinitis pigmentosa, retinal pathoconnectome 1 (RPC1). Preliminary data demonstrate an increase in endfoot entanglement in RPC1 when compared with RC1, and direct synaptic contact analysis of both connectomes is ongoing.