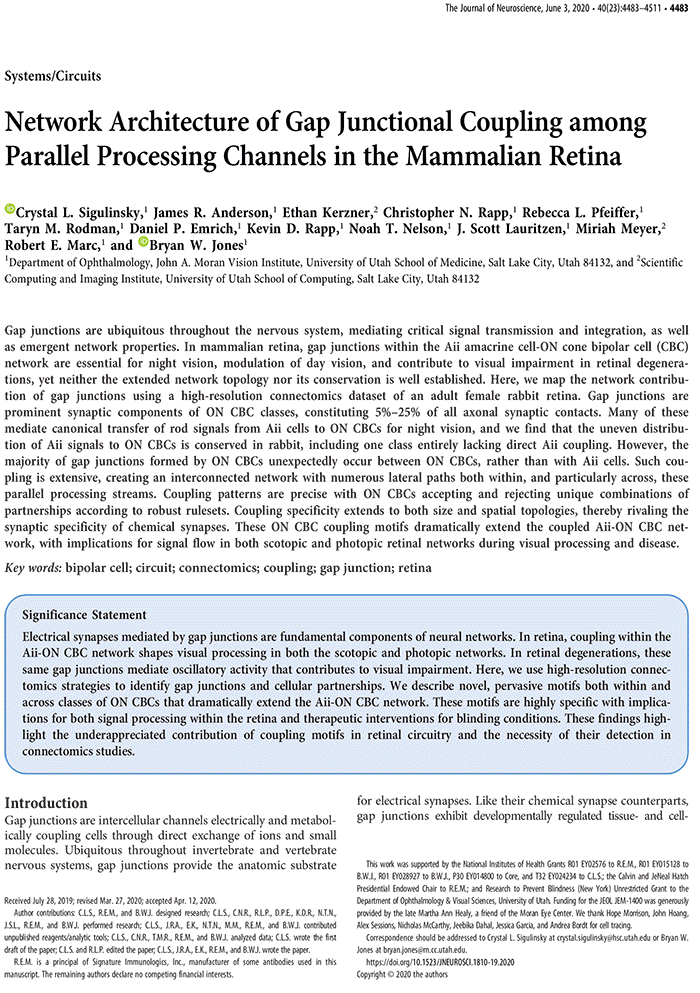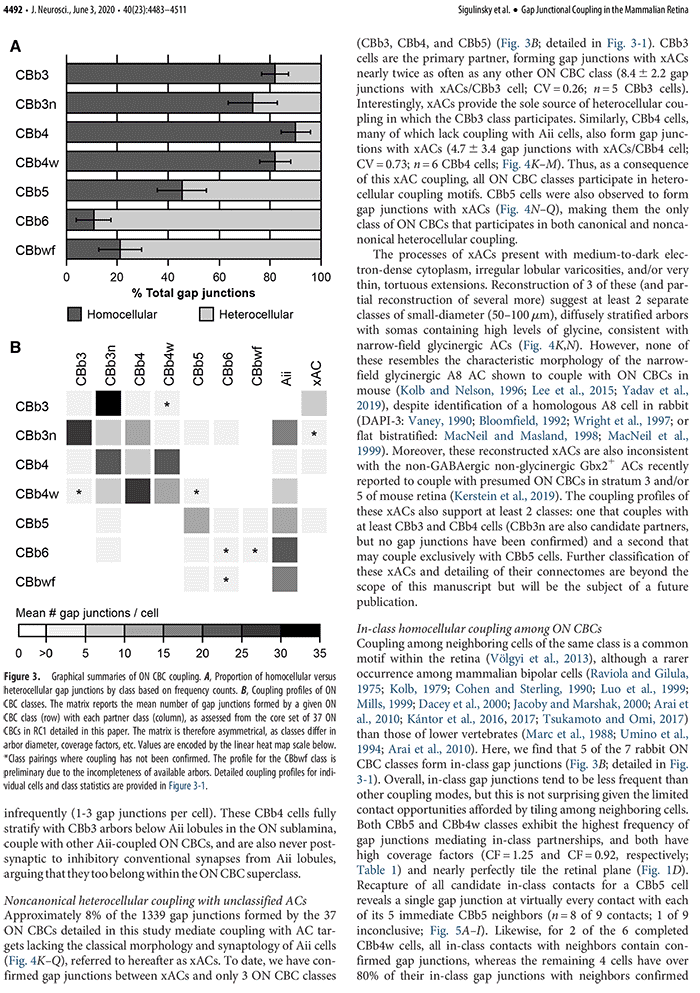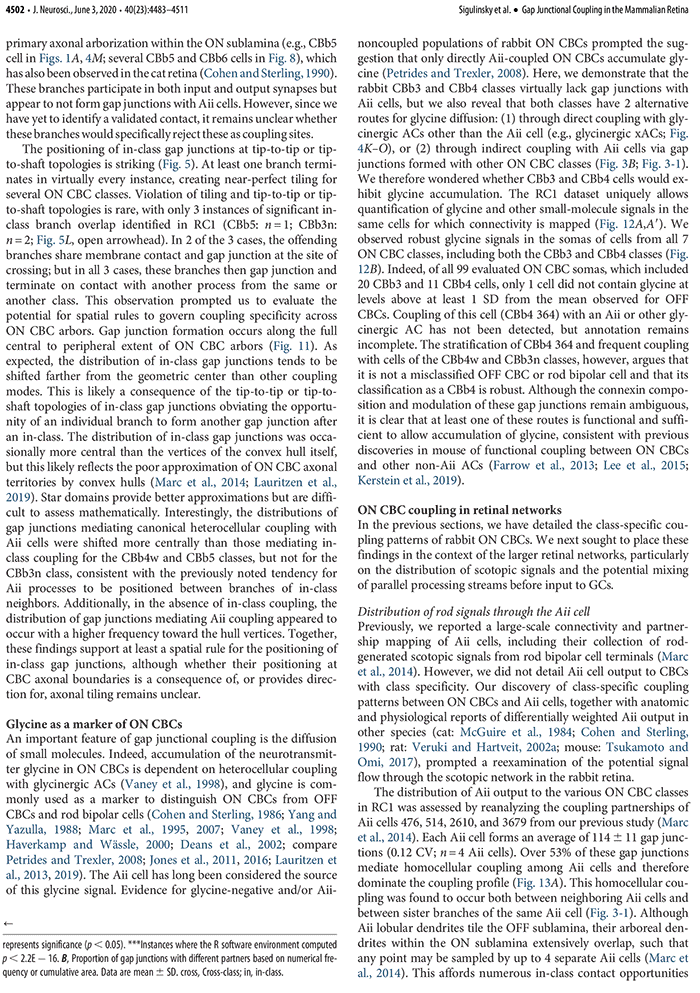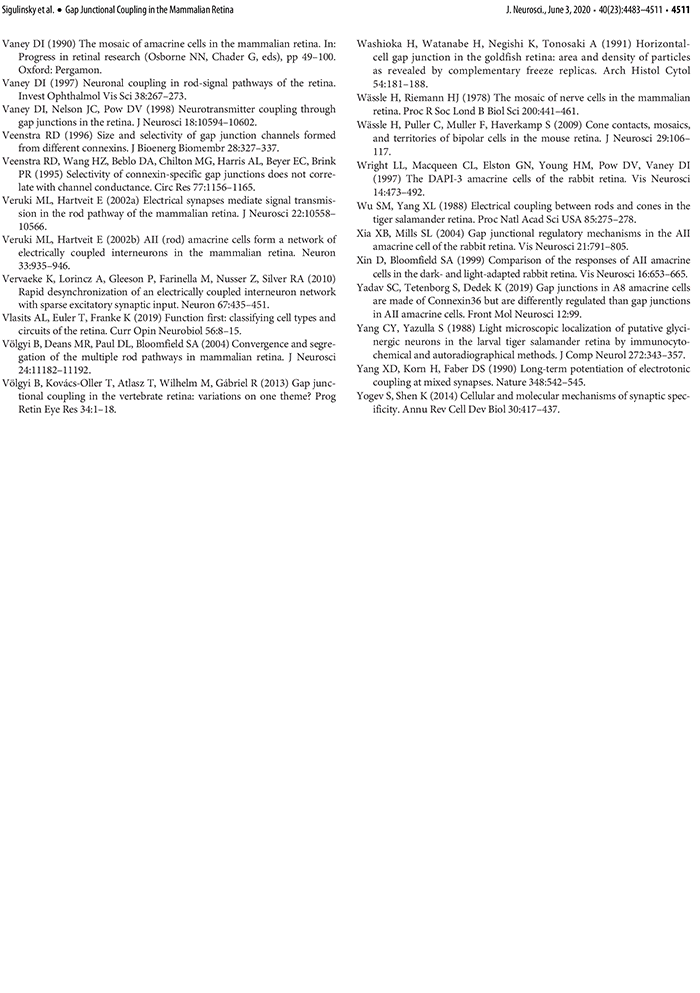
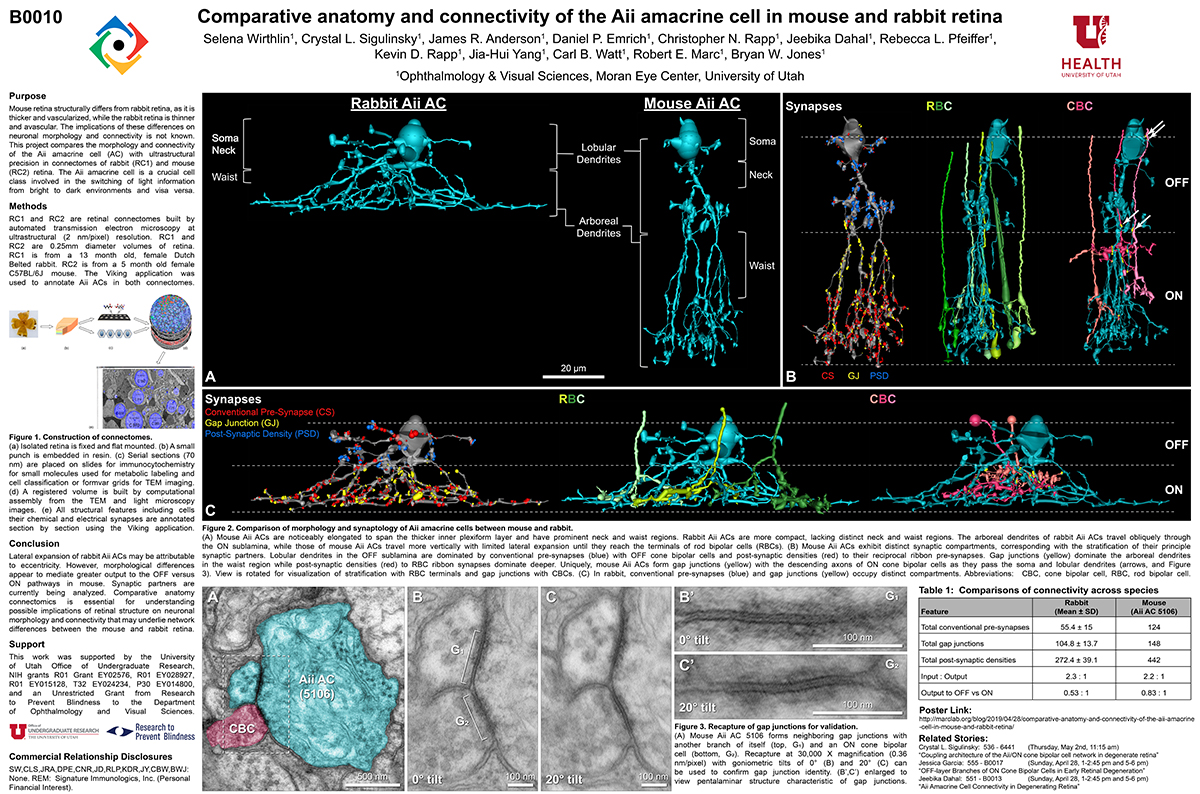
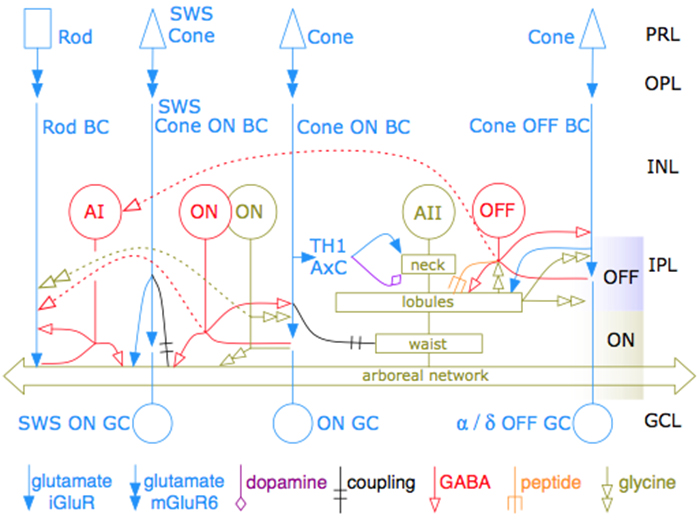
This talk was presented today, April 25th at the 2023 Association for Research in Vision and Opthalmology (ARVO) meetings in New Orleans, Louisiana by Crystal Sigulinsky as part of an ARVO Minisymposium organized by Bryan William Jones.
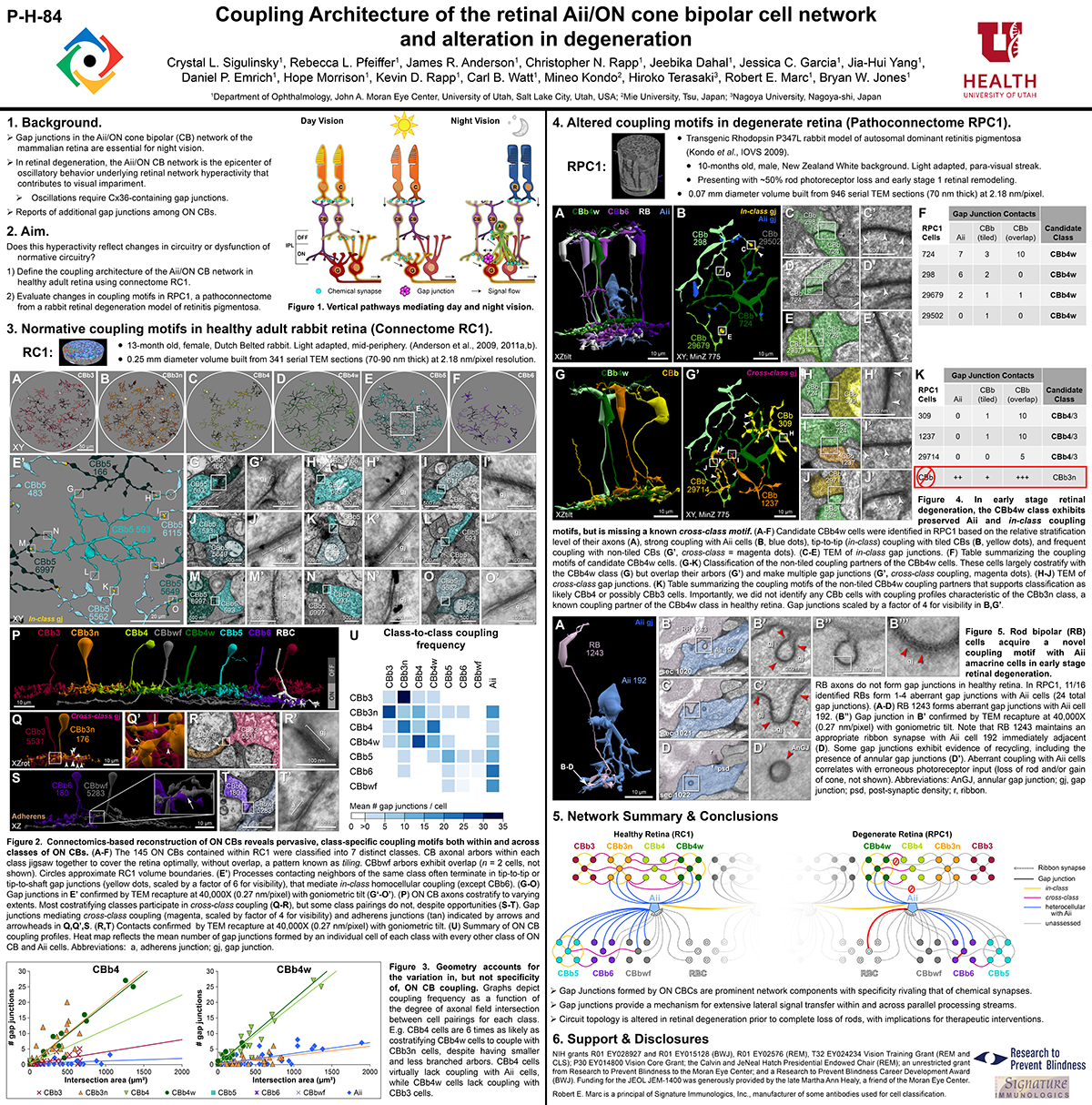
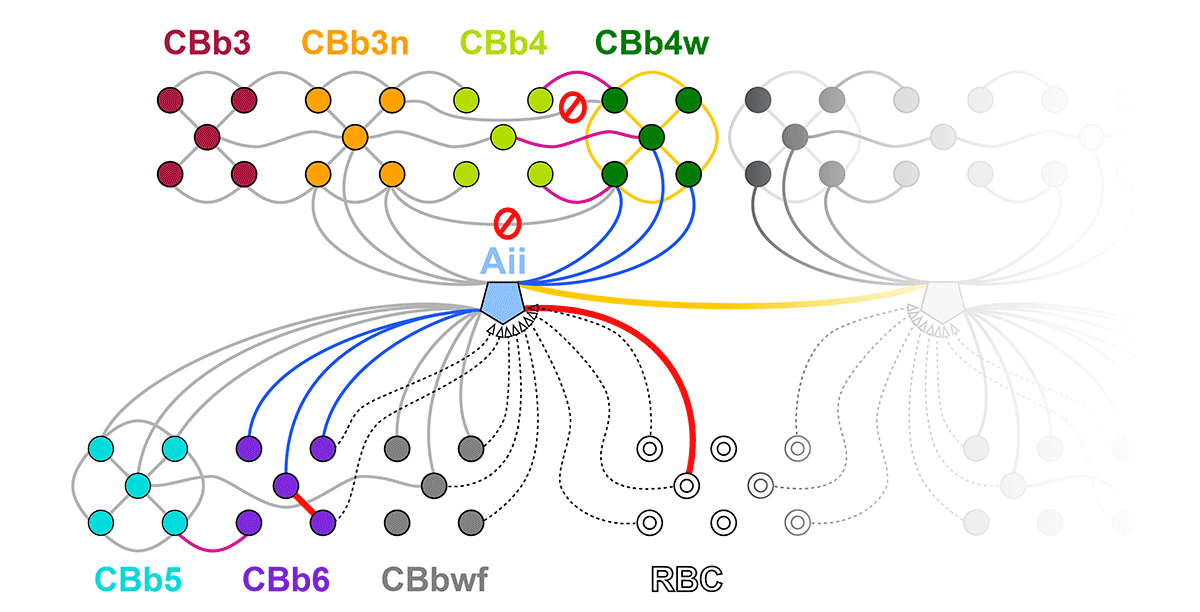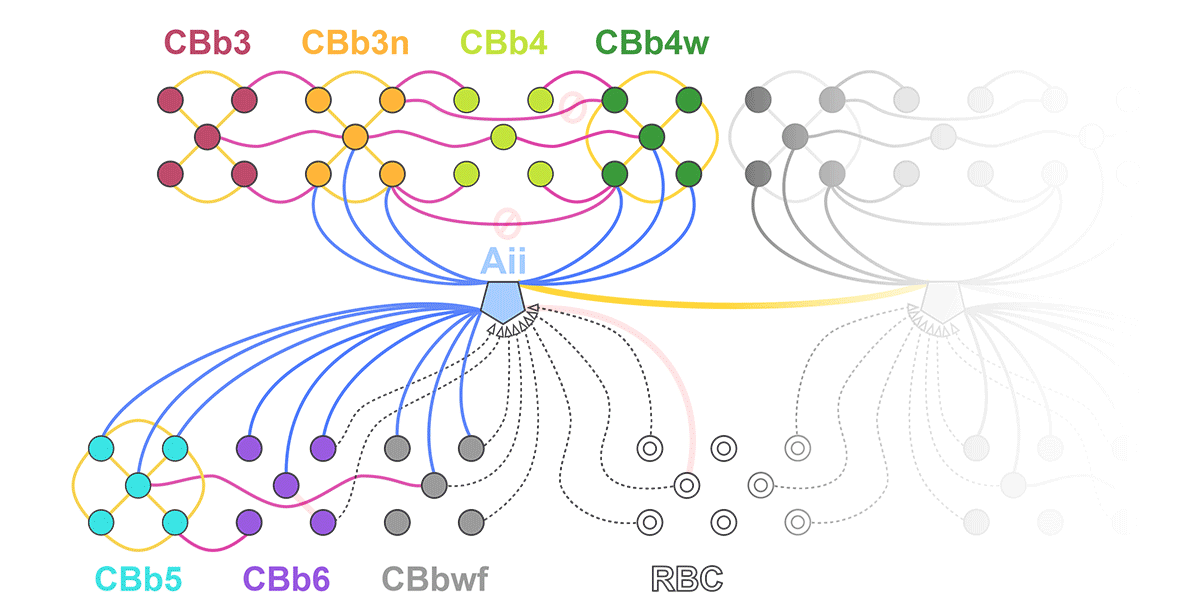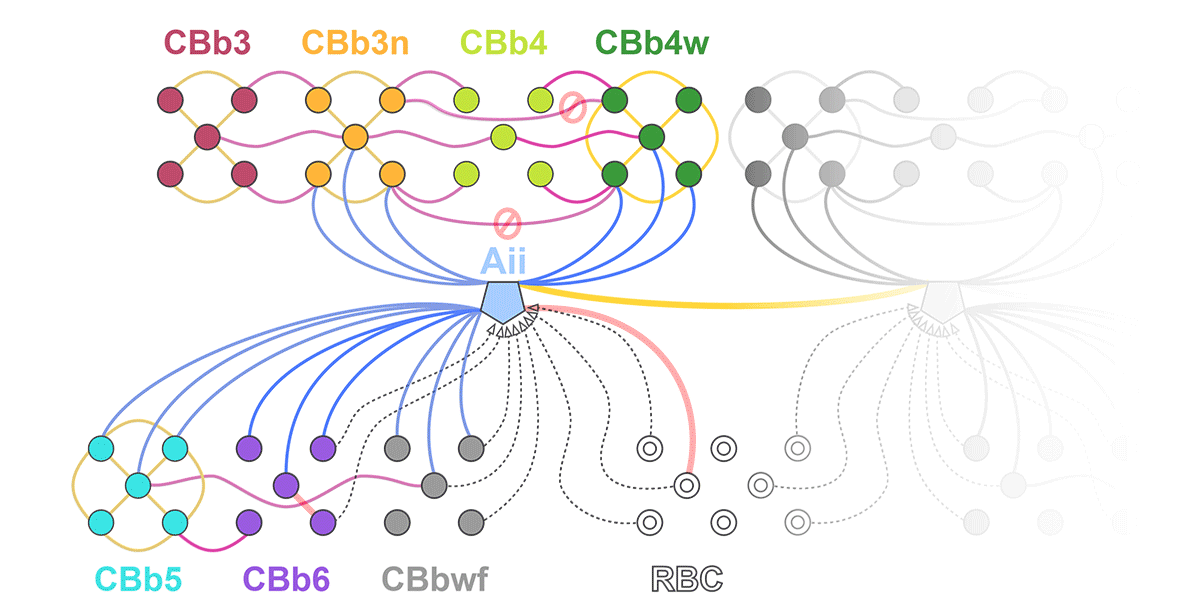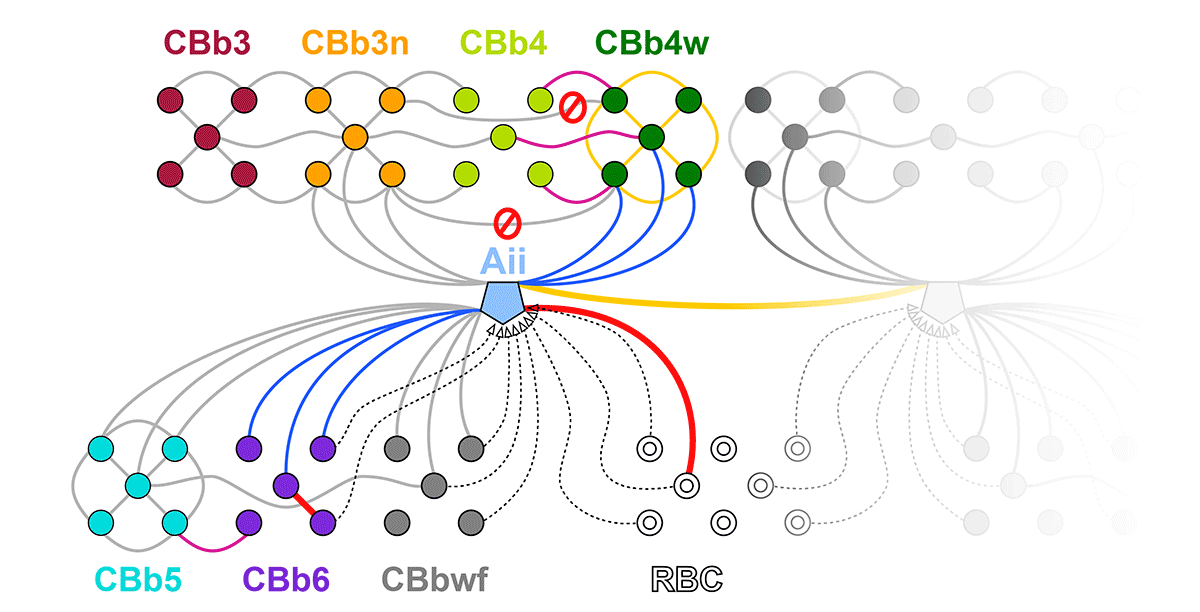
Abstract: Biomedical research relies heavily on animal models to study human disease and develop therapeutics. Understanding the architectural diversity in neural networks between humans and these model species is essential for choosing a relevant study model and interpreting conflicting results. Using comparative connectomics, we sought to map and compare the local neural network architecture of rabbit and mouse retinal Aii amacrine cells. This specialized narrow-field, multistratified, glycinergic interneuron has critical feedforward and feedback roles in both the photopic and scotopic retinal networks spanning the ON and OFF pathways, making it an ideal candidate for investigating species-specific differences in retinal networks. High-resolution, serial-section transmission electron microscopy (TEM) volumes of rabbit (RC1: female, 13- month, Dutch Belted) and mouse (RC2: female, 5-month, C57BL/6J) retinal tissue provided spatially-registered synaptic maps of Aii connectivity at directly comparable resolution and completeness. These reveal that despite species-specific morphologies, gross synaptology and compartmentalization appear conserved. Yet, rabbit and mouse Aii cells diverge in the weighting of their partnerships, most notably in their coupling profiles. Opposing biases in gap junction partnerships and their respective sizing rules indicate a greater relative output by mouse Aii cells to ON pathways than in rabbit. However, a unique topological conformation for a subset of conventional presynapses formed by Aii cell lobular dendrites with species-specific features and prevalence may influence signal output to specific partner classes within the OFF pathway and either nullify or exacerbate this difference in ON/OFF output. Additionally, rabbit Aii cells in RC1 showed greater Aii-Aii coupling than in mouse, which may suggest greater signal-to-noise compensation. Lastly, preliminary data suggest mouse Aii cells receive greater excitatory, but not inhibitory input/feedback from the OFF pathway than in rabbit. Together these data indicate that precise neural circuit architectures diverge between species and require detailed, comprehensive mapping to begin to dissect potential influence on signal flow.