This poster was presented today, May 2th at the 2016 Association for Research in Vision and Opthalmology (ARVO) meetings in Seattle, Washington by Rebecca L. Pfeiffer, Bryan W. Jones, and Robert E. Marc.
Posterboard #: D0246
Abstract Number: 2256 – D0246
Author Block: Rebecca L. Pfeiffer1,2 , Bryan W. Jones1,2 , Robert E. Marc1,2
1 Ophthalmology, University of Utah, Salt Lake City, Utah, United States; 2 Interdepartmental Program in Neuroscience, University of Utah, Salt Lake City, Utah, United States
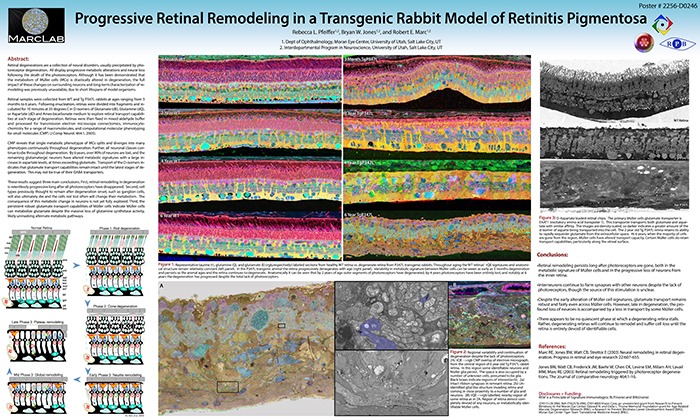
Purpose:Retinal degenerations are a collection of neural disorders, usually precipitated by photoreceptor degeneration. All display progressive metabolic alterations and neural loss following the death of the photoreceptors. Although it has been demonstrated that the metabolism of Müller cells (MCs) is drastically altered in degeneration, the full impact of these changes on surrounding neurons and long-term characterization of remodeling was previously unavailable, due to short lifespans of model organisms.
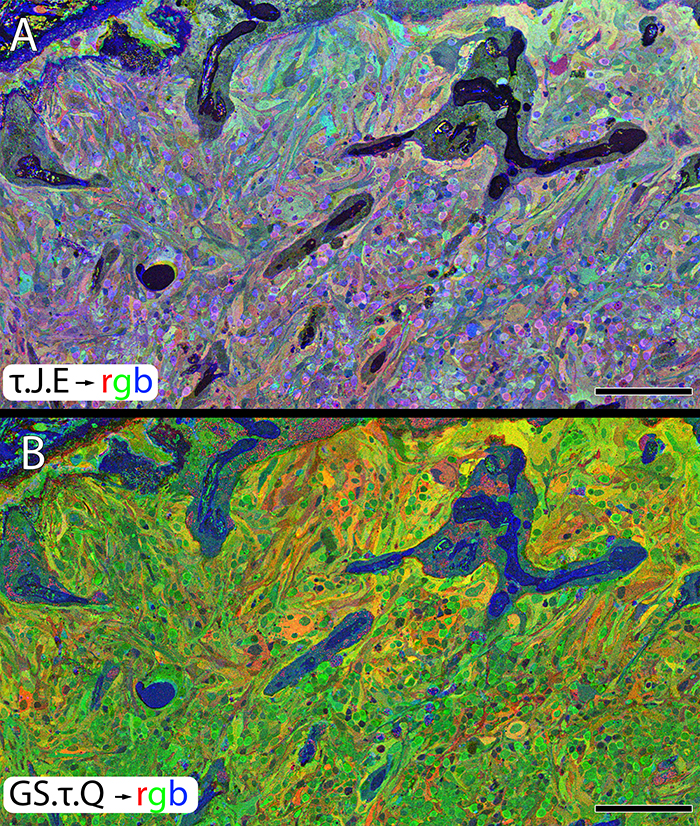
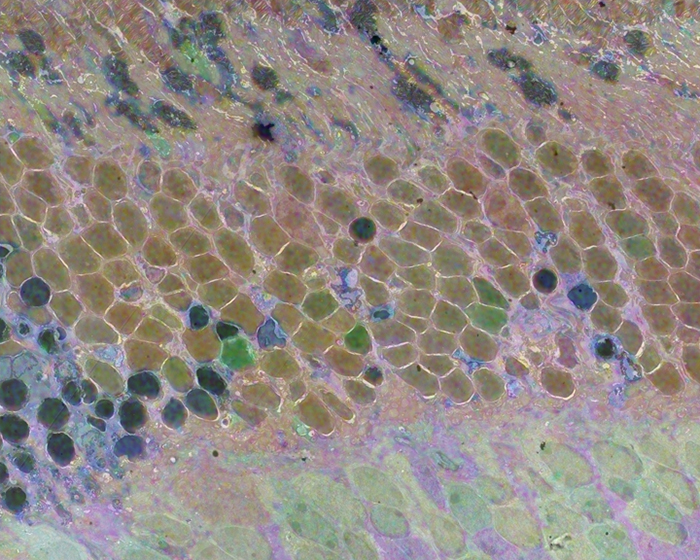
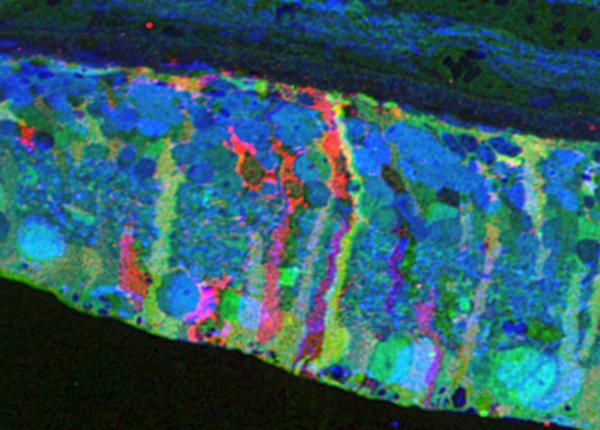
Methods:Retinal samples were collected from WT and Tg P347L rabbits at ages ranging from 3 months to 6 years. Following enucleation, retinas were divided into fragments and incubated for 10 minutes at 35 degrees C in D-isomers of Glutamate (dE), Glutamine (dQ), or Aspartate (dD) and Ames-bicarbonate medium to explore retinal transport capabilities at each stage of degeneration. Retinas were then fixed in mixed aldehyde buffer and processed for transmission electron microscope connectomics, immunocytochemistry for a range of macromolecules, and computational molecular phenotyping for small molecules (CMP) (J Comp Neurol. 464:1, 2003).
Results:CMP reveals that single metabolic phenotype of MCs splits and diverges into many phenotypes continuously throughout degeneration. Further, all neuronal classes continue to die throughout degeneration. By 6 years, over 90% of neurons are lost, and the remaining glutamatergic neurons have altered metabolic signatures with a large increase in aspartate levels, at times exceeding glutamate. Transport of the D-isomers indicates that glutamate transport capabilities remain intact until the latest stages of degeneration. This may not be true of their GABA transporters.
Conclusions:These results suggest three main conclusions. First, retinal remodeling in degeneration is relentlessly progressive long after all photoreceptors have disappeared. Second, cell types previously thought to remain after degeneration onset, such as ganglion cells, will also ultimately die and the cells not lost often will change their metabolism. The consequence of this metabolic change in neurons is not yet fully explored. Third, the persistent robust glutamate transport capabilities of Müller cells indicate Müller cells can metabolize glutamate despite the massive loss of glutamine synthetase activity, likely unmasking alternate metabolic pathways.