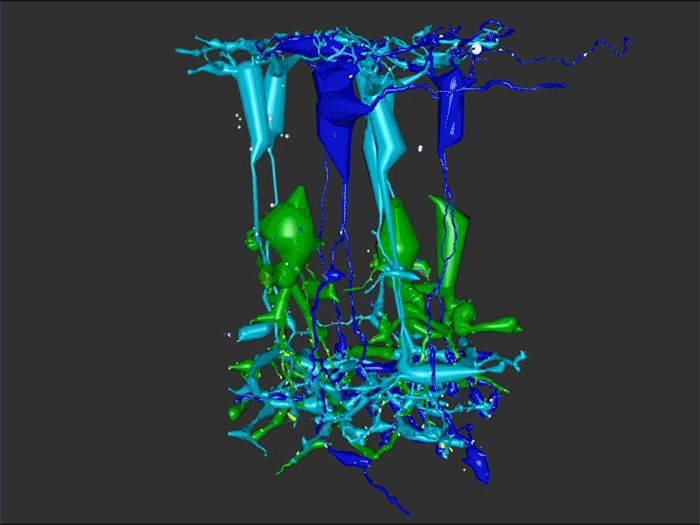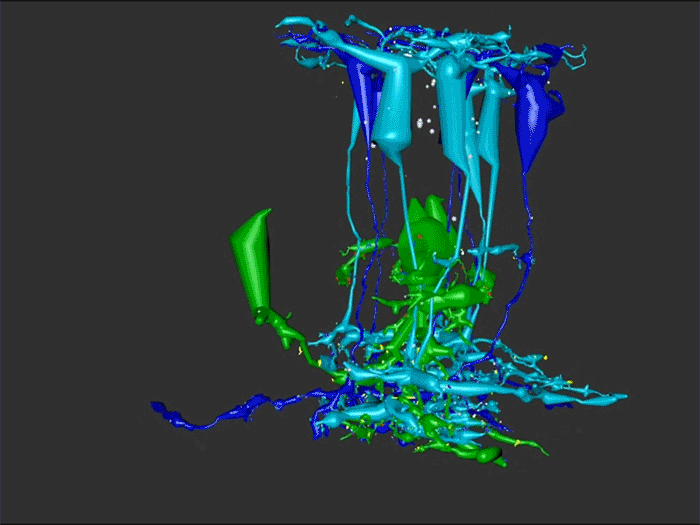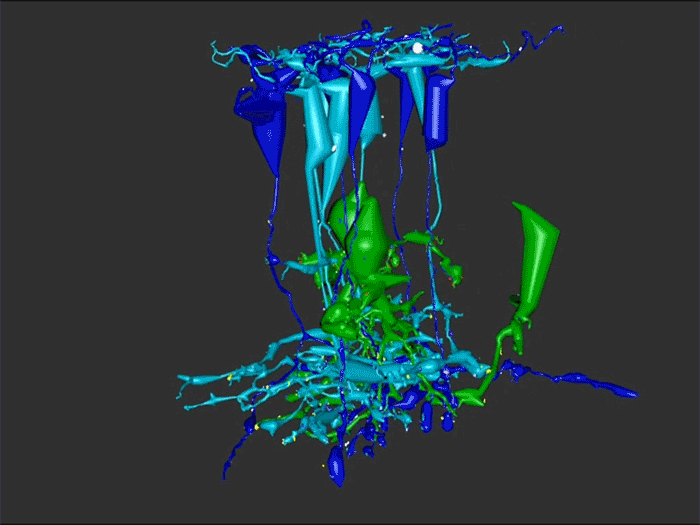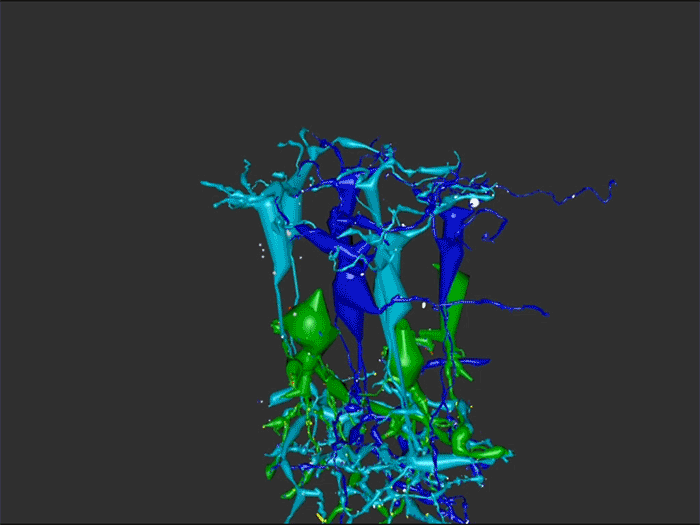
One of the undergraduates in the lab, Jessica Garcia is seen here annotating conectomes in the lab prior to her presentation at this years ARVO conference. Jessica came to us by way of service with the US Navy, and in our lab is exploring the OFF-layer branches of ON cone bipolar cells in early retinal degeneration. This is important work as we are unraveling how circuitry in the retina changes in neurodegenerative disease.
Ryan Initiative for Macular Research 2019
Last week I attended the Ryan Initiative for Macular Research meeting at the Beckman Center down in Los Angeles. This was my first time attending the meeting as I’ve not traditionally been an AMD scientist. Though we’ve been working more and more in the field, applying what we’ve learned through the study of other retinal degenerative diseases.
I gotta say this feels like a bit of a victory of sorts as we’ve been advocating more attention to the inner retina in retinal degenerative disease for a while now. Our work is better known in the field of retinitis pigmentosa, but we’ve published work showing that AMD behaves just like retinitis pigmentosa which is incredibly similar to CNS neurodegenerative disease. This is a perspective we will be pushing harder in the very near future, including a position that we should be using retina as a model for understanding CNS disease.
There are more photos of the meeting over on Jonesblog.
New Job Posting: Lab Technician
We have a new job posting for a technician in the lab.
The Marclab for Connectomics is seeking a highly motivated and energetic laboratory technician to work in ophthalmology research exploring retinal circuitry in the department of Ophthalmology and Visual Sciences. Salary and benefits will be commensurate with qualifications.
The laboratory technician will handle and process retinal tissues for light and transmission electron microscopy. This includes histological processing, sectioning, immunohistochemistry, staining of electron microscopy grids, operation of light and electron microscopes, and working with computational processing to “build” digital datasets; will also work with rabbits to perform OCT and ERG measurements. Must be able to work well in a team.
Bachelor’s degree in biology, chemistry, or other scientific disciplines in a related field or equivalency required; knowledge of complex laboratory techniques, equipment, terminology, materials and substances. Skilled in the use of laboratory equipment, ability to create and analyze statistical calculations and prepare reports required; and demonstrated human relations and effective communication skills also required. OSHA training and certification in animal care and use or the willingness to complete the certification may be required by some departments.
Applicants must demonstrate the potential ability to perform the essential functions of the job as outlined in the position description.
Minimum qualifications for this position is a bachelors degree in bio-sciences. Will train for laboratory specific demands, unique to this position.
Power To New JEM-1400
After having retired out old Hitachi transmission electron microscope, we are currently in the process of installing a new @JEOLUSA JEOL JEM-1400 transmission electron microscope to supplement our existing JEM-1400, and we have power!
Looking forward to first transmitted electrons.
Simple Super-Resolution Microscopy
Super-resolution microscopy is a pretty big thing right now. But there is more than one way to get super-resolution microscopy results. There are a variety of approaches, most involving expensive new microscopes that preclude many scientists from participating in science that allows them to ask certain questions. However, if they have access to a standard transmission election microscope and have antibodies that are glutaraldehyde tolerant, they can participate and ask questions that allow them to get around some of the inherent limitations imposed by physics.
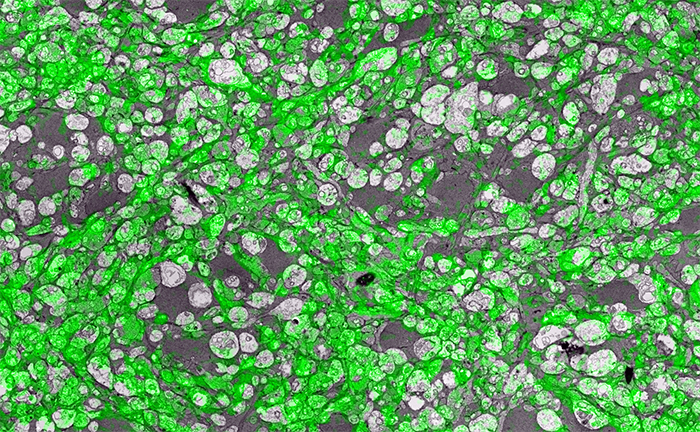
In the image above for example, we have GABA labeling in green superimposed upon ultrastructural data showing us *which* processes in the inner plexiform layer of the retina are GABAergic. Many of these processes are smaller than the wavelength of light.
There are multiple ways to get here of course with some very expensive microscopes offering dual light and electron microscopy approaches and yet other microscopes offering purely optical based solutions. However, this is cheap and easy and accessible to many with the basic electron microscopy resources. Robert Marc first used this approach in back in 2000, and we subsequently used it for quite a bit of work for my Ph.D. dissertation in 2003, and notably in this paper. It is also an integral technique associated with our connectomics efforts.
That said, I’ll need at some point soon to find the resources to get a traditional optical super-resolution microscopy solution to answer some questions we have in the lab on neural degenerative disease.
Photographic Ode To An Electron Microscope
It is time to say goodbye to a workhorse that has been a part of a tremendous amount of retinal science to make room for a new instrument that will help us expand our workflow. See more over on Jonesblog.
JCN Retinal Special Issue I: Mammals
Our paper Rod-cone crossover connectome of mammalian bipolar cells has been republished in a special issue of The Journal Of Comparative Neurology, Retinal Special Issue I: Mammals.
Merry Christmas / Happy Holidays 2018
Heterocellular Coupling Between Amacrine Cells and Ganglion Cells
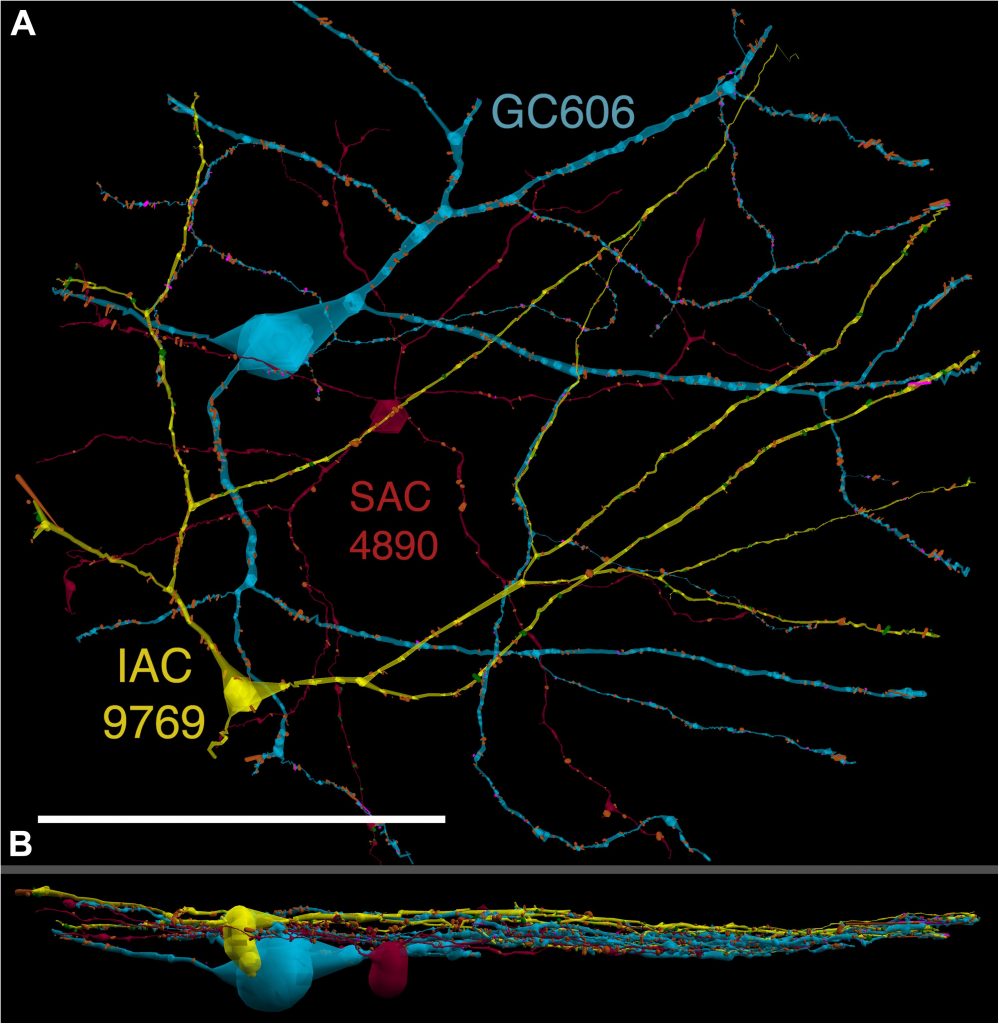
We have a new paper out In Frontiers in Neural Circuits, Heterocellular Coupling Between Amacrine Cells and Ganglion Cells. This manuscript preprint was published in BioRxiv.
Authors: Robert E. Marc, Crystal Lynn Sigulinsky, Rebecca L. Pfeiffer, Daniel Emrich, James Russel Anderson and Bryan William Jones.
Abstract: All superclasses of retinal neurons, including bipolar cells (BCs), amacrine cells (ACs) and ganglion cells (GCs), display gap junctional coupling. However, coupling varies extensively by class. Heterocellular AC coupling is common in many mammalian GC classes. Yet, the topology and functions of coupling networks remains largely undefined. GCs are the least frequent superclass in the inner plexiform layer and the gap junctions mediating GC-to-AC coupling (GC::AC) are sparsely arrayed amidst large cohorts of homocellular AC::AC, BC::BC, GC::GC and heterocellular AC::BC gap junctions. Here, we report quantitative coupling for identified GCs in retinal connectome 1 (RC1), a high resolution (2 nm) transmission electron microscopy-based volume of rabbit retina. These reveal that most GC gap junctions in RC1 are suboptical. GC classes lack direct cross-class homocellular coupling with other GCs, despite opportunities via direct membrane contact, while OFF alpha GCs and transient ON directionally selective (DS) GCs are strongly coupled to distinct AC cohorts. Integrated small molecule immunocytochemistry identifies these as GABAergic ACs (γ+ ACs). Multi-hop synaptic queries of RC1 connectome further profile these coupled γ+ ACs. Notably, OFF alpha GCs couple to OFF γ+ ACs and transient ON DS GCs couple to ON γ+ ACs, including a large interstitial amacrine cell, revealing matched ON/OFF photic drive polarities within coupled networks. Furthermore, BC input to these γ+ ACs is tightly matched to the GCs with which they couple. Evaluation of the coupled versus inhibitory targets of the γ+ ACs reveals that in both ON and OFF coupled GC networks these ACs are presynaptic to GC classes that are different than the classes with which they couple. These heterocellular coupling patterns provide a potential mechanism for an excited GC to indirectly inhibit nearby GCs of different classes. Similarly, coupled γ+ ACs engaged in feedback networks can leverage the additional gain of BC synapses in shaping the signaling of downstream targets based on their own selective coupling with GCs. A consequence of coupling is intercellular fluxes of small molecules. GC::AC coupling involves primarily γ+ cells, likely resulting in GABA diffusion into GCs. Surveying GABA signatures in the GC layer across diverse species suggests the majority of vertebrate retinas engage in GC::γ+ AC coupling.
Rod Bipolar Cell Networks in Early Retinal Remodeling
Rebecca Pfeiffer, a post-doc in the laboratory presented her work on “Rod Bipolar Cell Networks in Early Retinal Remodeling” as a platform presentation at the ISER 2018 meeting in Belfast, Northern Ireland.
Authors: Rebecca Pfeiffer, James R. Anderson, Daniel P. Emrich, Jeebika Dahal, Crystal L Sigulinsky, Hope AB Morrison, Jia-Hui Yang, Carl B. Watt, Kevin D. Rapp, Jessica C Garcia, Mineo Kondo, Hiroko Terasaki, Robert E. Marc, and Bryan W. Jones.
Abstract: Retinal remodeling is a form of negative plasticity that occurs as a consequence of retinal degenerative diseases. Part of retinal remodeling involves anomalous sprouting of processes, termed neurites. The synaptic structures and partners of the neurites are not yet defined, leading to uncertainty about the consistency of network motifs between healthy and degenerate retina. Our goal is to map out the identities and network relationships of bipolar cell networks using a connectomics strategy. Retinal connectomes or ultrastructural maps of neuronal connectivity have substantially contributed to our understanding of retinal network topology, providing ground truth against which pathological network topologies can be evaluated. We have generated the first pathoconnectome (RPC1), or connectome of pathological tissues, of early retinal remodeling at 2nm/pixel, and are currently investigating the impact of remodeling on network architecture.
The tissue for RPC1 was obtained from a 10mo transgenic P347L rabbit model of autosomal dominant retinitis pigmentosa. Tissue was fixed in mixed aldehydes, osmicated, dehydrated, embedded in epon resin, and sectioned at 70nm. Serial sections were placed on grids, stained, and imaged using a JEOL JEM-1400 TEM using SerialEM software. Every 30th section was reserved for computational molecular phenotyping (CMP), and probed for small molecules: glutamate, glutamine, glycine, GABA, taurine, glutathione; or TEM compatible proteins GFAP and GS. The pathoconnectome volume is explored and annotated using the Viking software suite.
RPC1 was selected as an example of early retinal remodeling, demonstrating Muller cell hypertrophy, metabolic dysregulation, and degeneration of rod outer segments, indicating phase 1 remodeling and neuronal sprouting. We have observed the presence of both cone pedicles and rod spherules within the OPL to be synaptically active with neurites from some rod bipolar cells forming functional synapses with both rod spherules and cone pedicles. These rod bipolar cells also exhibit structurally altered ribbon synapses. We are currently evaluating network motifs and comparing them to networks established from our previous connectome, RC1, generated from a healthy rabbit.
These findings allow us to evaluate and analyze the impact of retinal remodeling on retinal networks which may have important implications for therapeutic interventions being developed which rely on inner retina network integrity.