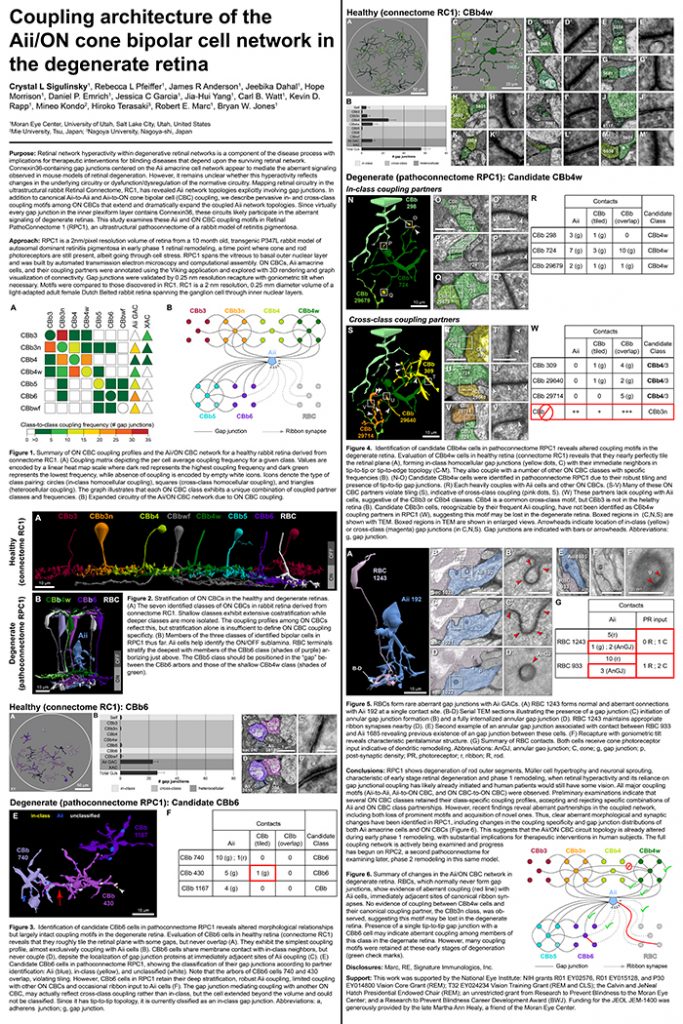
This abstract was presented today, April 8th at the 2019 Association for Research in Vision and Opthalmology (ARVO) meetings in Vancouver, Canada by Jeebika Dahal, Rebecca L. Pfeiffer, Crystal L. Sigulinsky, James R. Anderson, Daniel P. Emrich, Hope Morrison, Jessica C. Garcia, Kevin D. Rapp, Jia-Hui Yang, Carl B. Watt, Mineo Kondo, Hiroko Terasaki, Robert E. Marc and Bryan W. Jones.
Full resolution version here.
Purpose
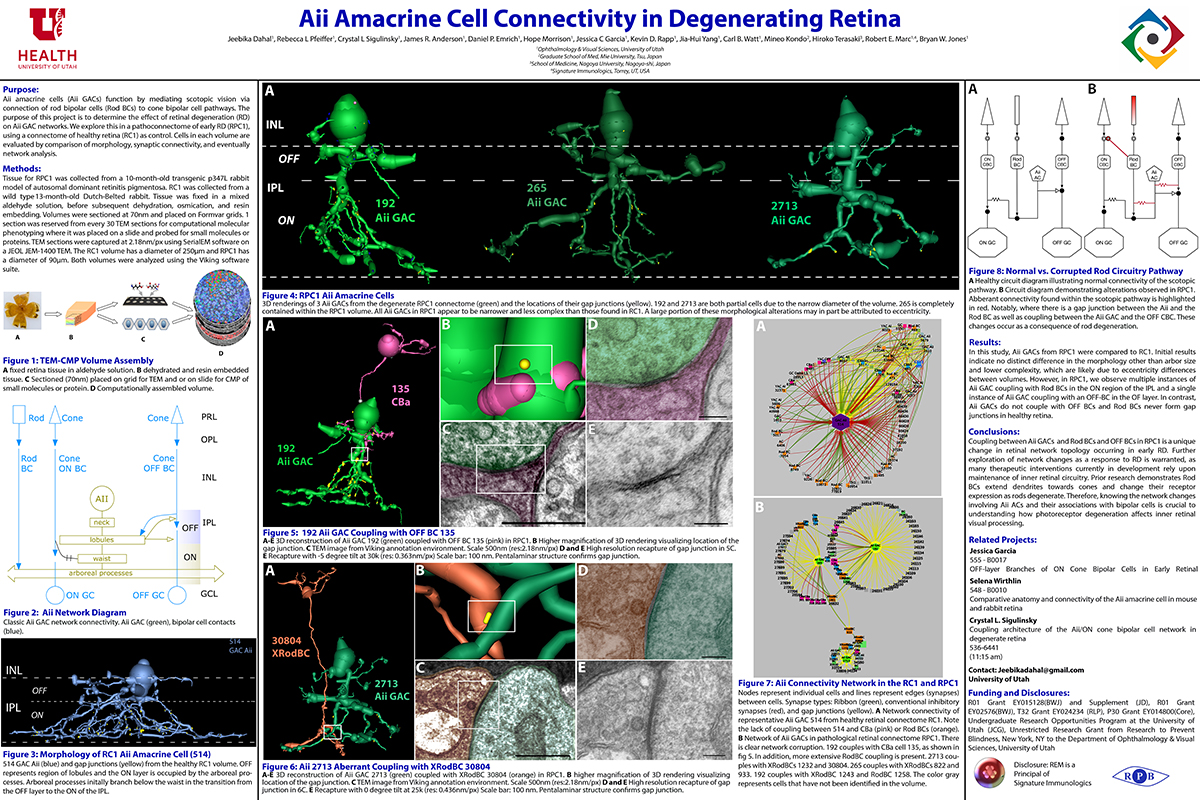
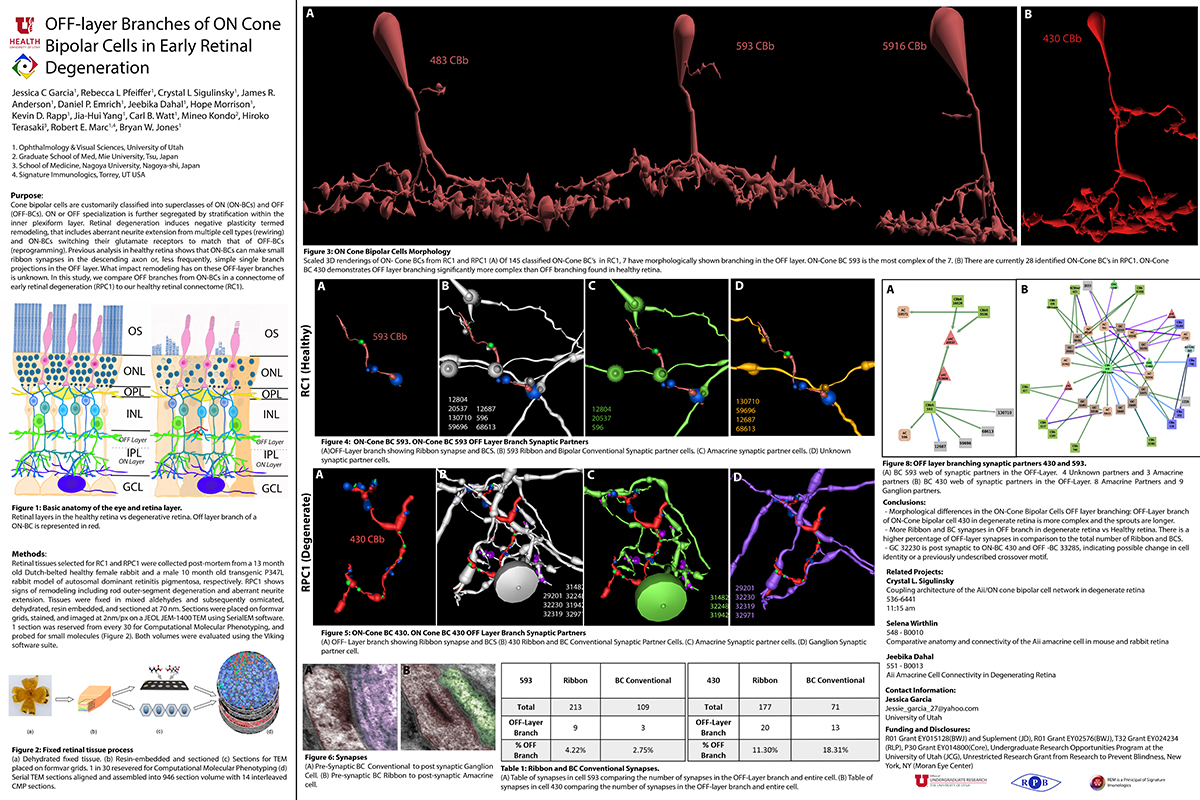
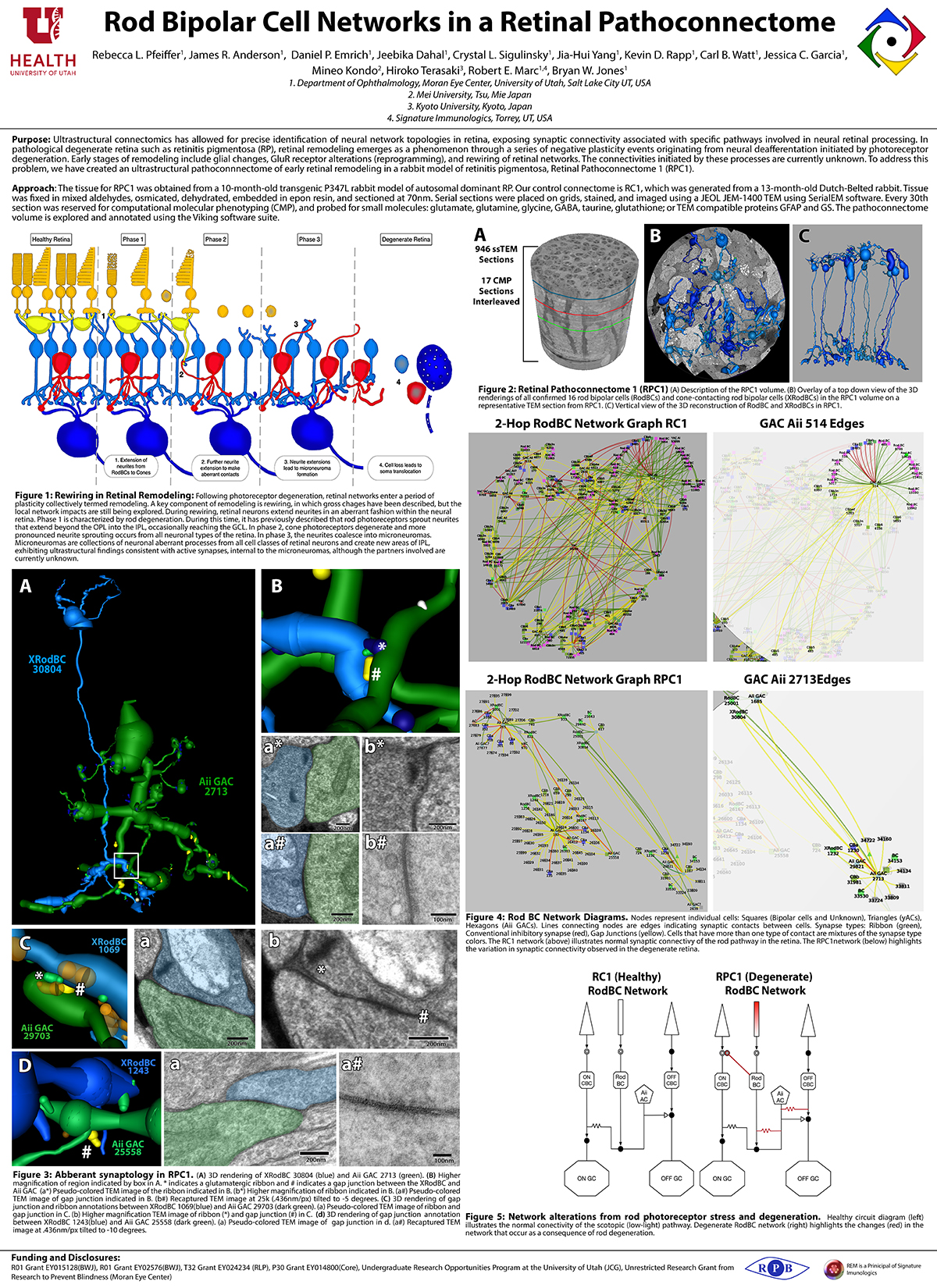
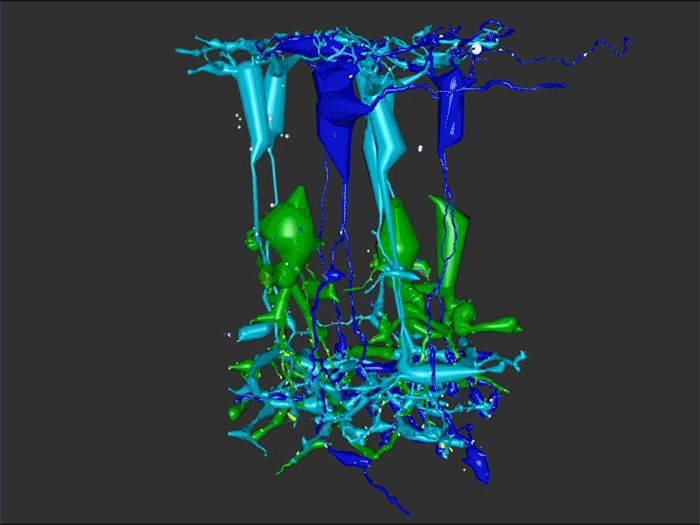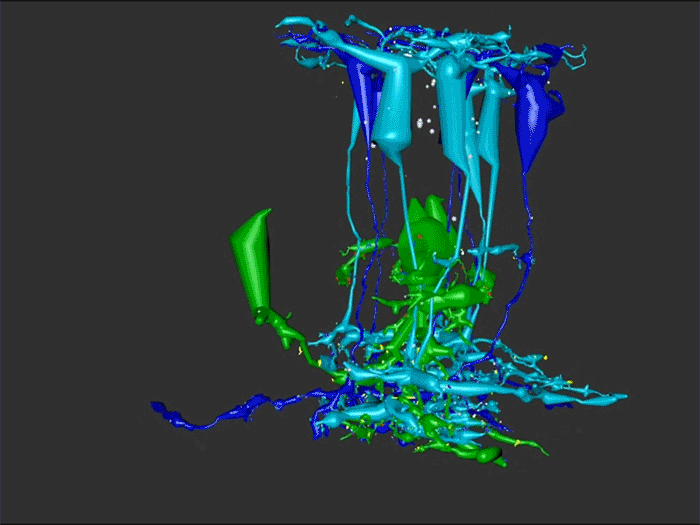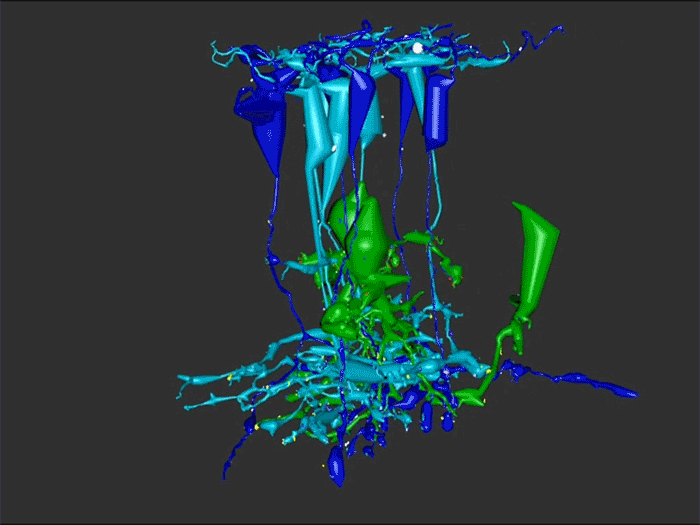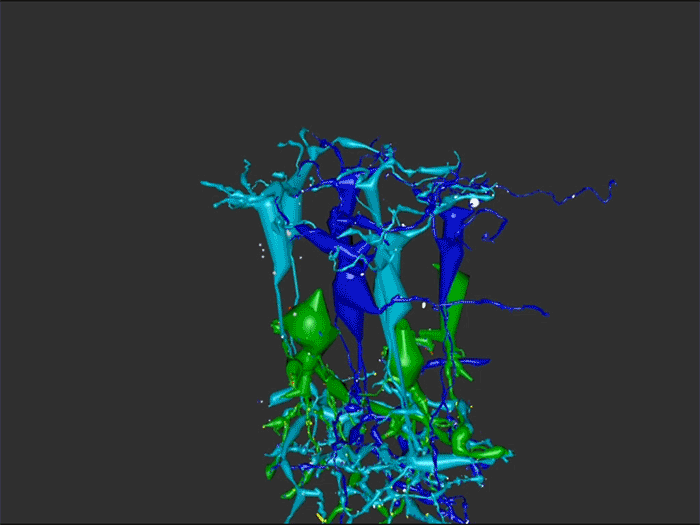
Aii amacrine cells (Aii ACs) function in mediating scotopic vision via connection of rod bipolar cells (Rod BCs) to cone bipolar cell pathways. The purpose of this project is to determine the effect of retinal degeneration (RD) on Aii AC networks. We explore this in a pathoconnectome of early RD (RPC1), using a connectome of healthy retina (RC1) as control. Cells in each volume are evaluated by comparison of morphology, synaptic connectivity, and eventually network analysis.
Methods
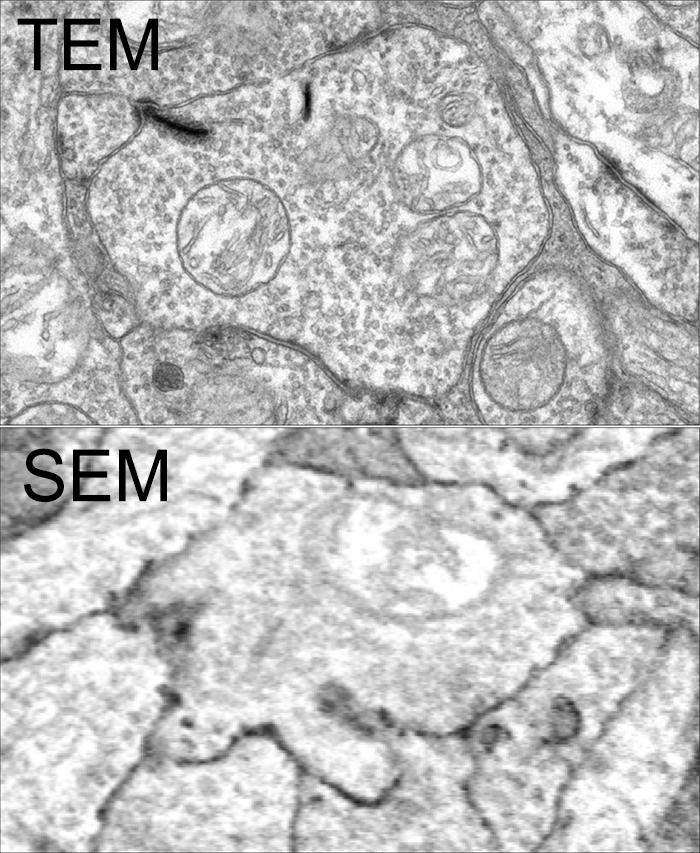
Tissue for RPC1 was collected from a 10 month old transgenic p347L rabbit model of autosomal dominant retinitis pigmentosa. RC1 was collected from a 13 month old Dutch-Belted rabbit, with no indications of degeneration. Tissue was fixed in a mixed aldehyde solution, before subsequent dehydration, osmication, and resin embedding. Volumes were sectioned at 70nm (RPC1) and 90nm (RC1) and placed on formvar grids. 1 section was reserved from every 30 TEM sections for computational molecular phenotyping where it was placed on a slide and probed for small molecules or proteins. TEM sections were captured at 2.18nm/px using SerialEM software on a JEOL JEM-1400 TEM. The RC1 volume has a diameter of 250µm and RPC1 has a diameter of 90µm. Both volumes were analyzed using the Viking software suite.
Results
In this study, Aii ACs from RPC1 were compared to RC1. Initial results indicate no distinct difference in the morphology other than arbor size, which are likely due to eccentricity differences between volumes. However, in RPC1, we observe multiple instances of Aii AC coupling with Rod BCs in the ON region of the IPL. In contrast, Rod BCs never form gap junctions in healthy retina.
Conclusions
Coupling between Aii ACs and Rod BCs in RPC1 is a unique change in retinal network topology occurring in early RD. Further exploration of network changes as a response to RD is warranted, as many therapeutic interventions currently in development rely upon maintenance of inner retinal circuitry. Prior research demonstrates Rod BCs extend dendrites towards cones and change their receptor expression as rods degenerate. Therefore, knowing the network changes involving Aii ACs and their associations with bipolar cells is crucial to understanding how photoreceptor degeneration affects inner retinal visual processing.