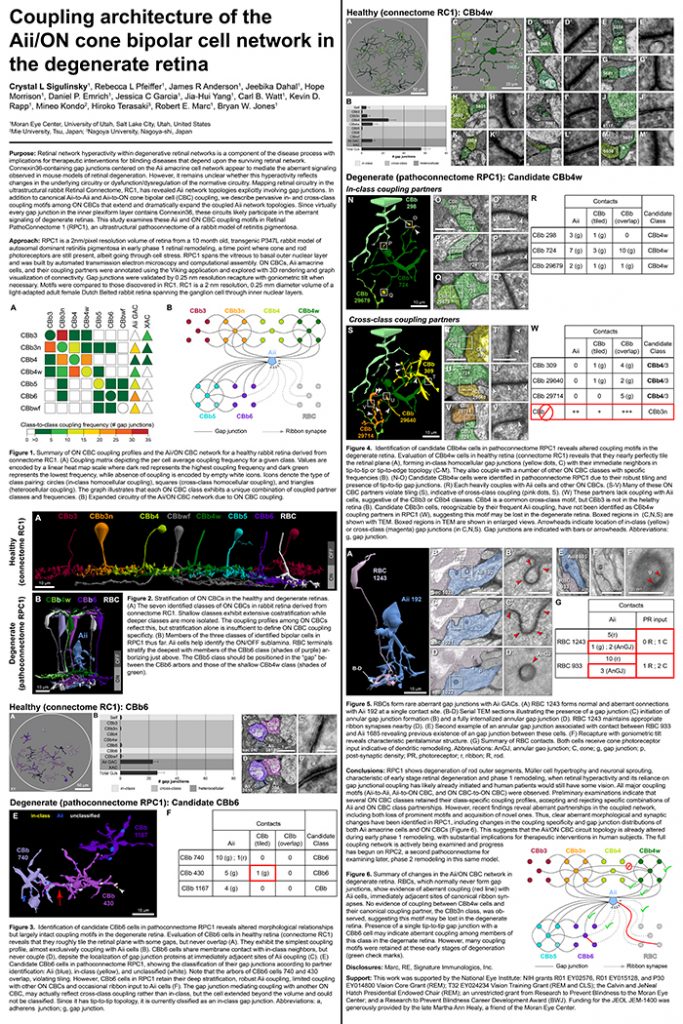
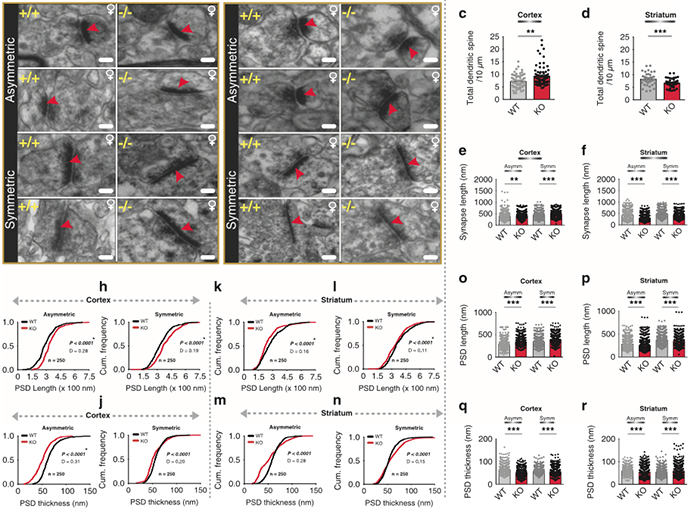
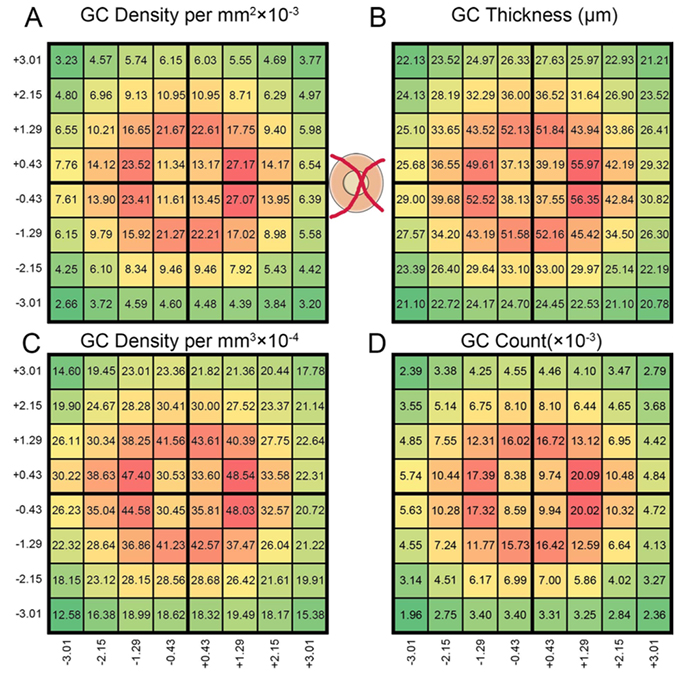
Crystal Sigulinsky, a post-doc in the lab, presented her work on “coupling architecture of the
Aii/ON cone bipolar cell network in the degenerate retina” at the RD2018 meeting in Killarney, Ireland today. Authors are: Crystal L Sigulinsky, Rebecca L Pfeiffer, James R Anderson, Jeebika Dahal, Hope Morrison, Daniel P. Emrich, Jessica C Garcia, Jia-Hui Yang, Carl B. Watt, Kevin D. Rapp, Mineo Kondo, Hiroko Terasaki, Robert E. Marc, and Bryan W. Jones.
Purpose: Retinal network hyperactivity within degenerative retinal networks is a component of the disease process with implications for therapeutic interventions for blinding diseases that depend upon the surviving retinal network. Connexin36-containing gap junctions centered on the Aii amacrine cell network appear to mediate the aberrant signaling observed in mouse models of retinal degeneration. However, it remains unclear whether this hyperactivity reflects changes in the underlying circuitry or dysfunction/dysregulation of the normative circuitry. Mapping retinal circuitry in the ultrastructural rabbit Retinal Connectome, RC1, has revealed Aii network topologies explicitly involving gap junctions. In addition to canonical Aii-to-Aii and Aii-to-ON cone bipolar cell (CBC) coupling, we describe pervasive in- and cross-class coupling motifs among ON CBCs that extend and dramatically expand the coupled Aii network topologies. Since virtually every gap junction in the inner plexiform layer contains Connexin36, these circuits likely participate in the aberrant signaling of degenerate retinas. This study examines these Aii and ON CBC coupling motifs in Retinal PathoConnectome 1 (RPC1), an ultrastructural pathoconnectome of a rabbit model of retinitis pigmentosa.
Approach: RPC1 is a 2nm/pixel resolution volume of retina from a 10 month old, transgenic P347L rabbit model of autosomal dominant retinitis pigmentosa in early phase 1 retinal remodeling, a time point where cone and rod photoreceptors are still present, albeit going through cell stress. RPC1 spans the vitreous to basal outer nuclear layer and was built by automated transmission electron microscopy and computational assembly. ON CBCs, Aii amacrine cells, and their coupling partners were annotated using the Viking application and explored with 3D rendering and graph visualization of connectivity. Gap junctions were validated by 0.25 nm resolution recapture with goniometric tilt when necessary. Motifs were compared to those discovered in RC1. RC1 is a 2 nm resolution, 0.25 mm diameter volume of a light-adapted adult female Dutch Belted rabbit retina spanning the ganglion cell through inner nuclear layers.
Conclusions: RPC1 shows degeneration of rod outer segments, Müller cell hypertrophy and neuronal sprouting, characteristic of early stage retinal degeneration and phase 1 remodeling, when retinal hyperactivity and its reliance on gap junctional coupling has likely already initiated and human patients would still have some vision. All major coupling motifs (Aii-to-Aii, Aii-to-ON CBC, and ON CBC-to-ON CBC) were observed. Preliminary examinations indicate that several ON CBC classes retained their class-specific coupling profiles, accepting and rejecting specific combinations of Aii and ON CBC class partnerships. However, recent findings reveal aberrant partnerships in the coupled network, including both loss of prominent motifs and acquisition of novel ones. Thus, clear aberrant morphological and synaptic changes have been identified in RPC1, including changes in the coupling specificity and gap junction distributions of both Aii amacrine cells and ON CBCs (Figure 6). This suggests that the Aii/ON CBC circuit topology is already altered during early phase 1 remodeling, with substantial implications for therapeutic interventions in human subjects. The full coupling network is actively being examined and progress has begun on RPC2, a second pathoconnectome for examining later, phase 2 remodeling in this same model.
An almost full size poster available here in pdf format.