This abstract was presented today at the 2014 Association for Research in Vision and Opthalmology (ARVO) meetings in Orlando, Florida by J Scott Lauritzen, Noah T. Nelson, Crystal L. Sigulinsky, Nathan Sherbotie, John Hoang, Rebecca L. Pfeiffer, James R. Anderson, Carl B. Watt, Bryan W. Jones and Robert E. Marc.
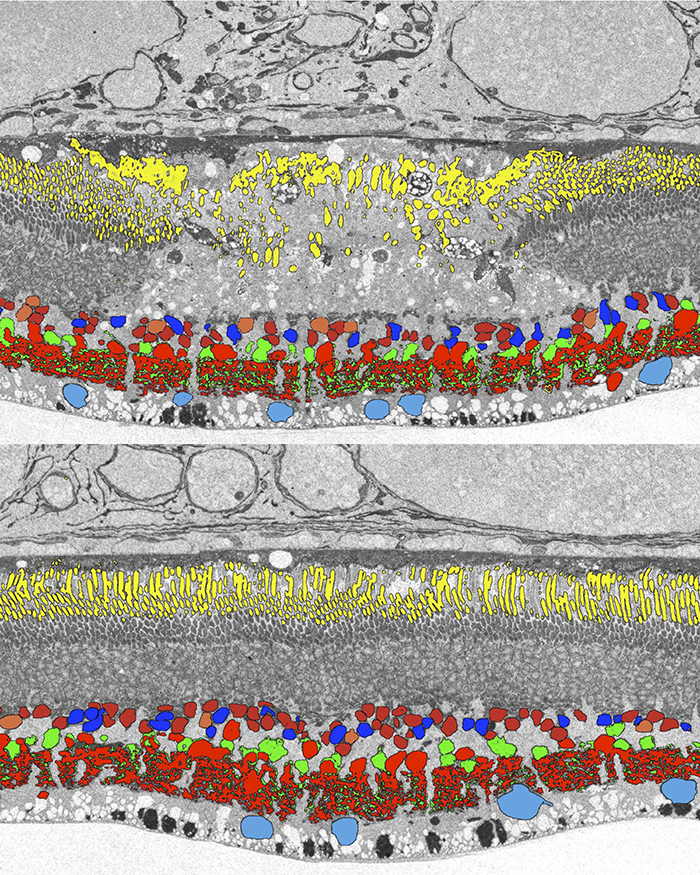
Purpose: Converging evidence suggests that large- and intermediate-scale neural networks throughout the nervous system exhibit small world’ design characterized by high local clustering of connections yet short path length between neuronal modules (Watts & Strogatz 1998 Nature; Sporns et al.2004 Trends in Cog Sci). It is suspected that this organizing principle scales to local networks (Ganmor et al. 2011 J Neurosci; Sporns 2006 BioSystems) but direct observation of synapses and local network topologies mediating small world design has not been achieved in any neuronal tissue. We sought direct evidence for synaptic and topological substrates that instantiate small world network architectures in the ON inner plexiform layer (IPL) of the rabbit retina. To test this we mined ≈ 200 ON cone bipolar cells (BCs) and ≈ 500 inhibitory amacrine cell (AC) processes in the ultrastructural rabbit retinal connectome (RC1).
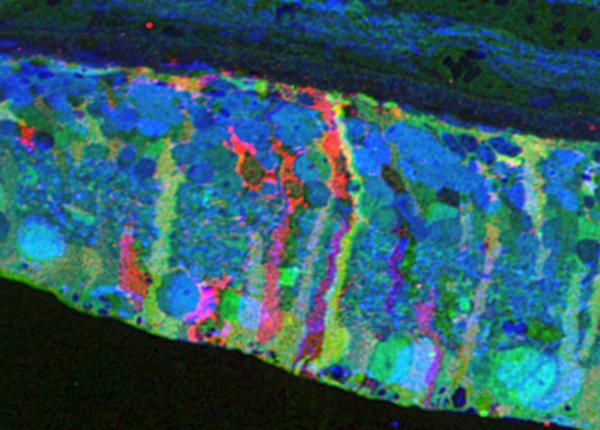
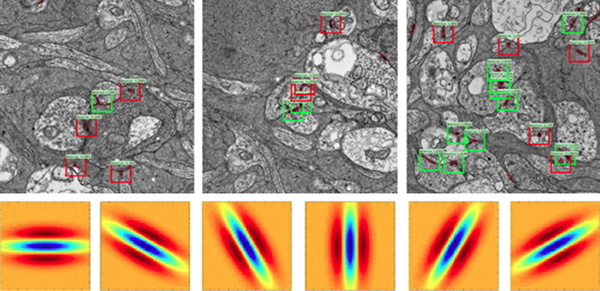
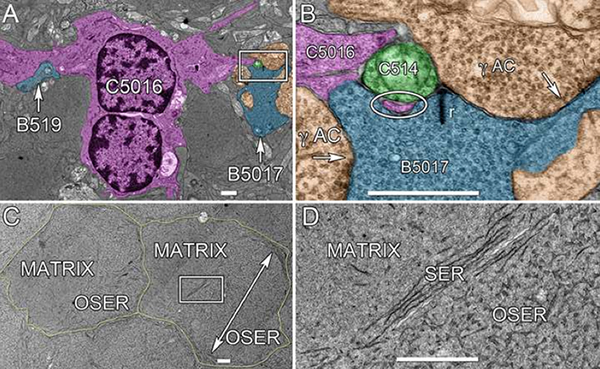
Methods: BC networks in RC1 were annotated with the Viking viewer and explored via graph visualization of connectivity and 3D rendering (Anderson et al. 2011 J Microscopy). Small molecule signals embedded in RC1 e.g. GABA glycine and L-glutamate combined with morphological reconstruction and connectivity analysis allow for robust cell classification. MacNeil et al. (2004 J Comp Neurol) BC classification scheme used for clarity.
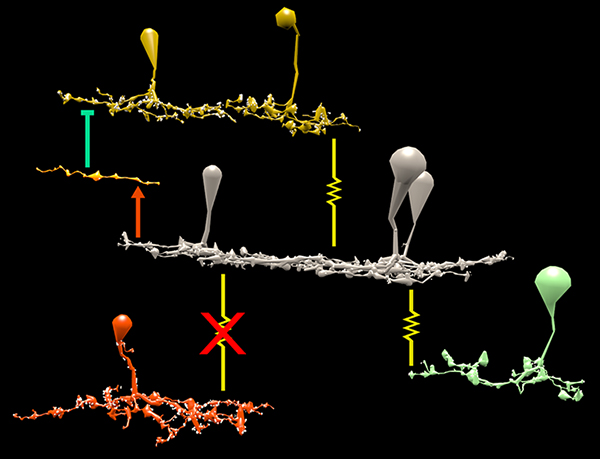
Results: Homocellular BC coupling (CBb3::CBb3 CBb4::CBb4 CBb5::CBb5) and within-class BC inhibitory networks (CBb3 → AC –| CBb3 CBb4 → AC –| CBb4 CBb5 → AC –| CBb5) in each ON IPL strata form laminar-specific functional sheets with high clustering coefficients. Heterocellular BC coupling (CBb3::CBb4 CBb4::CBb5 CBb3::CBb5) and cross-class BC inhibitory networks (CBb3 → AC –| CBb4 CBb4 → AC –| CBb3 CBb4 → AC –| CBb5 CBb5 → AC –| CBb4 CBb3 → AC –| CBb5 CBb5 → AC –| CBb3) establish short synaptic path lengths across all ON IPL laminae.
Conclusions: The retina contains a greater than expected number of synaptic hubs that multiplex parallel channels presynaptic to ganglion cells. The results validate a synaptic basis (ie. direct synaptic connectivity) and local network topology for the small world architecture indicated at larger scales providing neuroanatomical plausibility of this organization for local networks and are consistent with small world design as a fundamental organizing principle of neural networks on multiple spatial scales.
Support: NIH EY02576 (RM), NIH EY015128 (RM), NSF 0941717 (RM), NIH EY014800 Vision Core (RM), RPB CDA (BWJ), Thome AMD Grant (BWJ).