This abstract was presented July 1st at the 11th International Converence of the Metabolomics Society at the University of California, Davis by Felix Vazquez-Chona, Drew Ferrell, Bryan W. Jones and Robert E. Marc.
Metabolic dysregulation is an early hallmark of neurodegenerative diseases including Alzheimer’s disease and age-related macular degeneration. Mapping metabolic adaptation with cellular resolution and tissue- wide context is crucial to define networks regulating neuronal survival, cell death progression, and immune cell response.
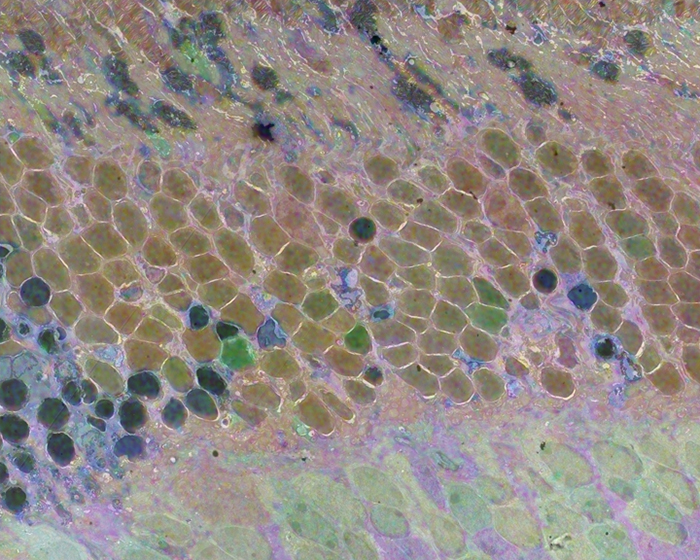
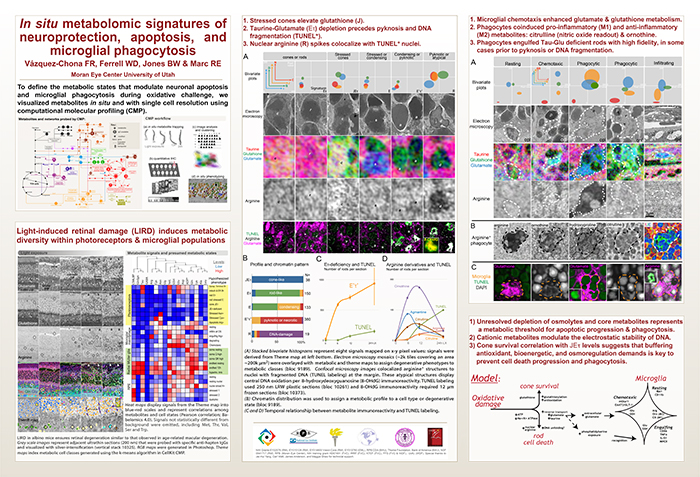
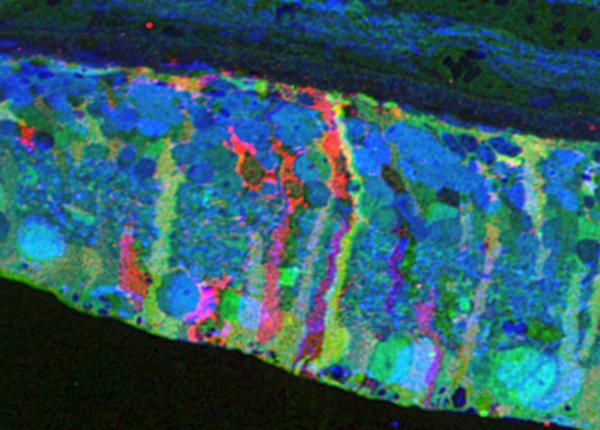
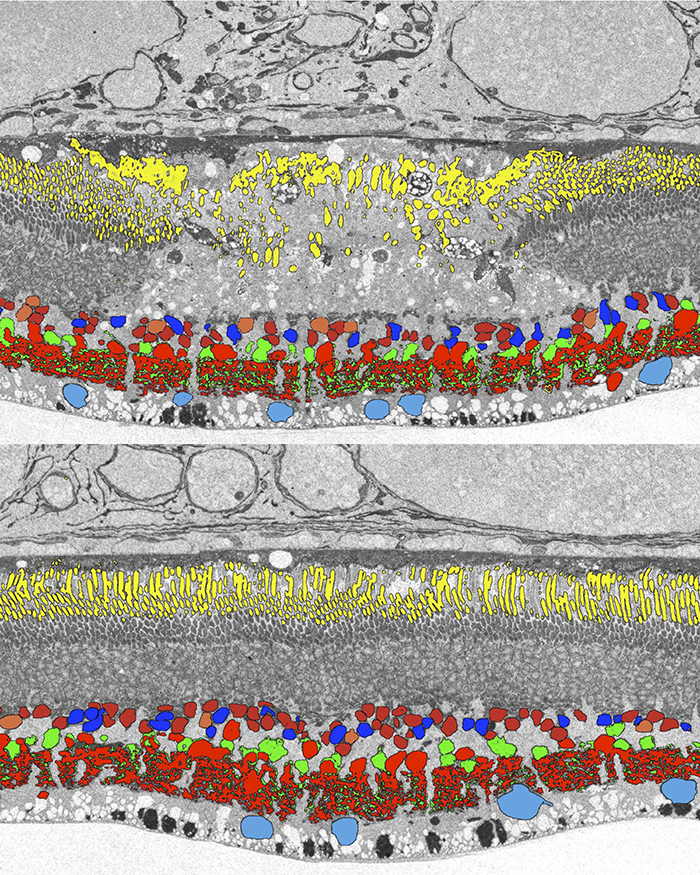
Computational Molecular Phenotyping (CMP) explores the amine metabolome (amino acids and amines). Technically, CMP metabolomics combines amine metabolite trapping, ultrathin microscopy (50-200 nm), immunodetection, pattern recognition, and clustering algorithms. Here we mapped the in situ distribution of over 30 core amine metabolites in retinal cells challenged by light-induced oxidative stress. Metabolomic profiles were phenotyped using ultrastructural, biochemical, and proteomic indices of oxidative stress.
CMP enabled precise visualization of >30 metabolites in every retinal cell. CMP resolved and phenotyped metabolomic profiles to specific degeneration and microglial functional states in the light-damaged retina. Cone photoreceptor survival correlated with enhanced antioxidant glutathione content. Rod photoreceptor apoptosis coincided with rapid depletion of organic osmolytes followed by nuclear import of cationic arginine metabolites. Delay in cell death increased necrosis and DNA damage-induced apoptosis. Microglial chemotaxis enhanced distinct signatures of glutamate and glutathione metabolism; whereas, phagocytosis coinduced classic (M1) and alternative (M2) arginine metabolites of macrophage activation.
CMP discovers and phenotypes cell classes, tracks cell state, and maps disease with single-cell resolution in any tissue or organism.