This abstract was presented today, Monday, April 30th at the 2018 Association for Research in Vision and Opthalmology (ARVO) meetings in Honolulu, Hawaii by Rebecca Pfeiffer, Robert E. Marc, James R. Anderson, Daniel P. Emrich, Carl B. Watt, Jia-Hui Yang, Kevin D. Rapp, Jeebika Dahal, Mineo Kondo, Hiroko Terasaki, and Bryan W. Jones.
Purpose:
Retinal remodeling is a consequence of retinal degenerative disease, during which neurons sprout new neurites whose synaptic structures and partners are not yet defined. Simultaneously during remodeling, Müller cells (MCs) undergo structural and metabolic changes, whose impact on surrounding neurons is an active area of research. Retinal connectomes have elucidated and validated fundamental networks. These data provide further classification of neuronal types and subtypes and a precise framework for modeling of retinal function, based on ground truth networks. The creation of the first pathoconnectome (RPC1), a connectome from pathological retinal tissue, provides the opportunity to determine connectivites between neurons, while simultaneously evaluating glial remodeling. Computational Molecular Phenotyping (CMP) embedded within the ultrastructure provides metabolic factors of pathologies.
Methods:
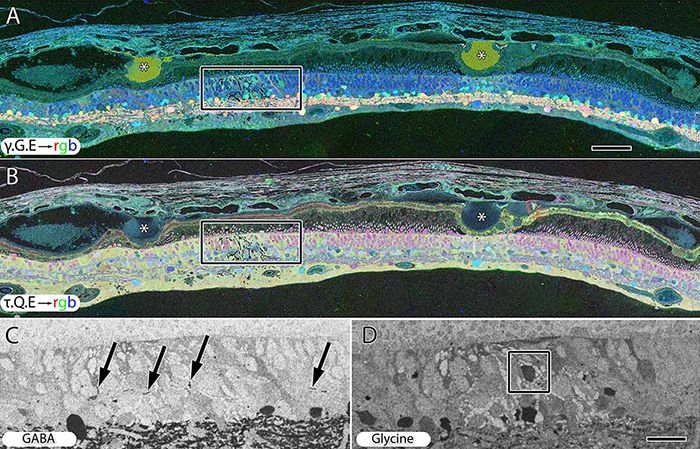
RPC1 was collected post-mortem from a 10mo TgP347L rabbit model of adRP, fixed in 1% FA, 2.5% GA, 3% sucrose, and 1mM MgSO4 in cacodylate buffer (pH 7.4). The tissue was osmicated, dehydrated, resin embedded, and sectioned at 70nm. Sections were placed on formvar grids, stained, and imaged on a JEOL JEM-1400 TEM using SerialEM. 1 section was reserved from every 30 section for CMP, where it was probed for small molecules: glutamate, glutamine, glycine, GABA, taurine, glutathione; or proteins GFAP and GS. RPC1 was evaluated using the Viking software suite.
Results:
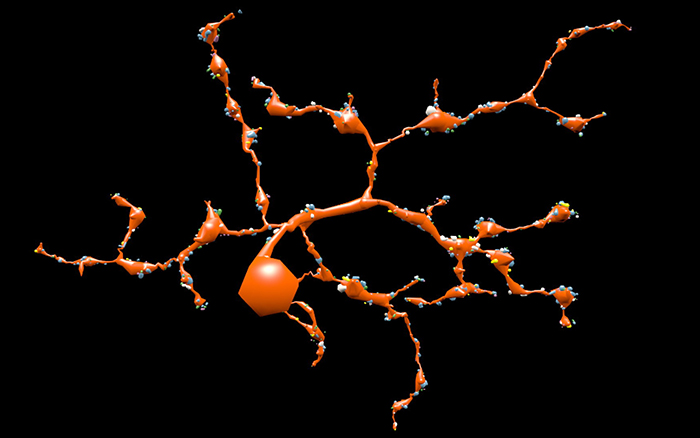
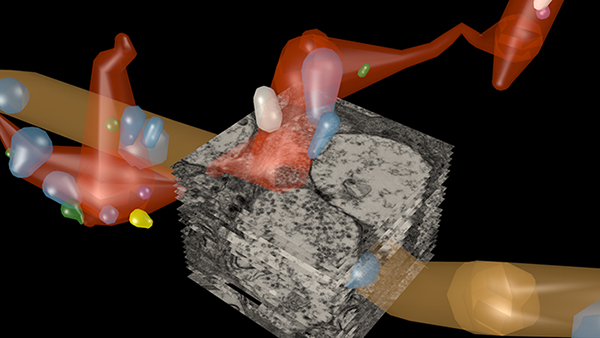
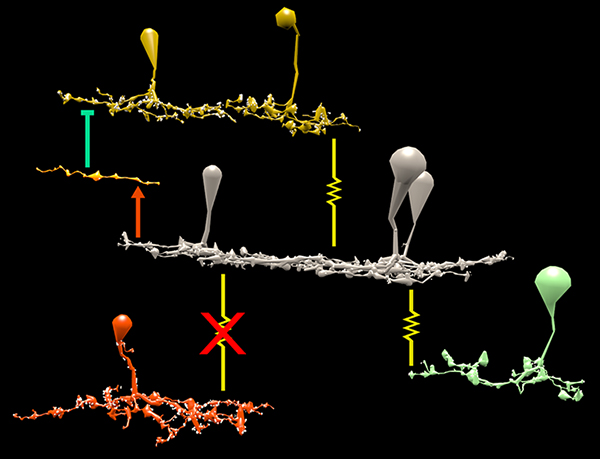
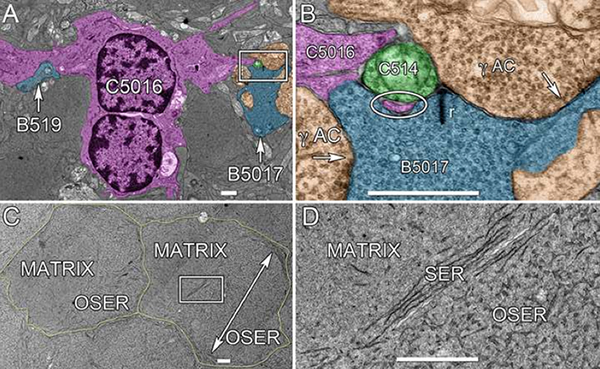
RPC1 was chosen based on early features of retinal degeneration/remodeling: degeneration of rod OS, MC hypertrophy, and neuronal sprouting. RPC1 consists of 948 serial sections spanning the ONL to the vitreous, with a diameter of 90µm. We find dendrites extending from rod bipolar cells to cone pedicles, originally described in light microscopy, and active synaptic contacts. We also see alterations of synaptic structure in the IPL, and MC morphological changes affecting surface to volume and neuron/glial relationships. Network motifs are being actively investigated.
Conclusions:
We observe many features of remodeling previously described using light microscopy, and confirm active synaptic contact. We also find synaptic structural features, not previously described. In addition, early evaluation of MC morphology demonstrates marked changes in MC shape and associations with nearby neurons and glia, which, combined with CMP, will be instrumental in understanding how MCs affect retinal remodeling.